Long-term results of forward intensity-modulated radiation therapy for patients with early-stage breast cancer
Article information
Abstract
Purpose
To observe long-term clinical outcomes for patients with early-stage breast cancer treated with forward intensity-modulated radiation therapy (IMRT), including local control and clinical toxicities.
Materials and Methods
We retrospectively analyzed a total of 214 patients with stage I-II breast cancer who were treated with breast conserving surgery followed by adjuvant breast radiation therapy between 2001 and 2008. All patients were treated using forward IMRT. The whole breast was irradiated to a dose of 50 to 50.4 Gy followed by an 8 to 12 Gy electron boost to the surgical bed.
Results
The median age was 46 years (range, 21 to 82 years) and the medial follow-up time was 7.3 years (range, 2.4 to 11.7 years). Stage T1 was 139 (65%) and T2 was 75 (35%), respectively. Ipsilateral breast recurrence was observed in 3 patients. The 5- and 10-year local control rates were 99.1% and 97.8%, respectively. The cosmetic outcome was evaluated according to the Harvard scale and 89.4% of patients were scored as excellent or good.
Conclusion
The whole breast radiation therapy as an adjuvant treatment using a forward IMRT technique showed excellent long-term local control as well as favorable outcomes of toxicity and cosmesis.
Introduction
Breast cancer is the second most commonly diagnosed cancer among women in Korea [1]. Standard treatment for early-stage breast cancer is breast conserving surgery followed by postoperative radiation therapy to the whole breast. This approach has resulted in comparable local control and survival rates after a mastectomy [2,3].
Conventional radiation therapy using opposite two tangential fields can result in substantial dose inhomogeneity even using wedges, especially in patients with large breasts [4,5]. This dose inhomogeneity is related to the increased risk of acute and late toxicity, such as moist desquamation and subcutaneous fibrosis and might compromise cosmetic outcome, which is the most important reason for breast conserving surgery [6-8].
To achieve more homogeneous dose distribution, breast intensity-modulated radiation therapy (IMRT) techniques have been studied in many previous studies. Several studies have shown dosimetric results that breast IMRT can improve the dose distribution [9,10]. For breast IMRT, both inverse and forward planning are available. Mihai et al. [11] compared the inverse and forward dose optimization algorithms on a prospective cohort of 30 patients. They reported that both algorithms were equivalent in removing the hot spots and no significant differences were detected between the two techniques.
There are limited studies reporting the clinical outcomes of breast IMRT techniques. Two randomized controlled trials and a retrospective trial have shown a beneficial effect of breast IMRT on acute and late toxicities [12-14]. To date, there is only one study which has evaluated the effect of IMRT on local control. In this retrospective study with 240 patients with stage 0-III breast cancer, McDonald et al. [15] reported that breast IMRT had significantly decreased acute dermatitis and long-term follow-up showed excellent local control similar to a cohort treated with conventional radiation therapy.
Since January 2001, we have been using forward IMRT to treat the patients with nearly all staged breast cancers. The aim of this study was to observe long-term clinical outcomes for patients with early-stage breast cancer treated with breast-conserving surgery followed by forward IMRT including local control and clinical toxicities.
Materials and Methods
1. Patients
Between January 2001 and February 2008, a total of 302 patients with stage I-II breast cancer were treated with breast-conserving surgery followed by radiation therapy in our institution. Men, patients with bilateral breast cancer, and those who had a previous history of ipsilateral breast cancer or other malignancies were excluded. Patients who were followed up for less than 24 months were also excluded. As a result, a total of 214 consecutive patients were included in this study. We retrospectively analyzed the medical records of the patients. Staging was performed according to the 7th edition of the American Joint Committee on Cancer.
2. Surgery and adjuvant therapy
Patients underwent breast-conserving surgery with or without axillary nodal dissection. All patients with positive sentinel node biopsy underwent axillary nodal dissection. Patients at substantial risk for distant metastasis who had a high grade tumor, lymphovascular invasion or lymph nodal involvement were offered adjuvant chemotherapy according to the surgeon's decision. Patients with hormone receptor positive breast cancer generally received adjuvant hormonal therapy.
3. Radiation therapy
For treatment planning, computed tomography (CT) simulation was done in the supine and arm-up position with a 3-mm slice thickness. Breast extent was defined clinically by the physician during simulation. Lead wires were placed on the sternal midline and mid-axillary line or 2 cm posterior to the breast. Also, the superior and inferior borders were marked allowing 2 cm margins from the breast tissue.
All patients were treated using the forward IMRT technique. The whole breast RT was given by a linear accelerator (PRIMUS; Siemens, Erlangen, German) of 6 or 10 MV to a dose of 50.4 Gy in 1.8 Gy fraction sizes. The prescription was given to the normalization point. Most patients were treated using a 6-MV photon beam, but for patients with a large separation of the chest wall, a mixed 6-MV and 10-MV photon beam was used. Two opposing tangential beams were aligned to pass through both medial and lateral radiopaque markers. And then one or two segments were added to each tangential beam to block out the hot spots receiving more than 105% of the prescribed dose as well as the lung volume (Fig. 1). In addition, segments were added to compensate for the cold spots in the breast volume in a few cases.
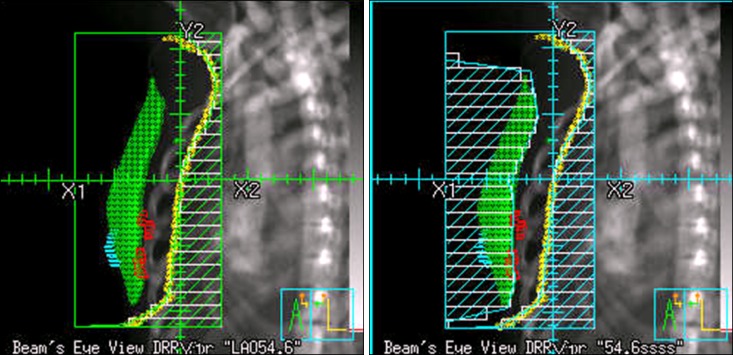
Dose distribution of forward intensity-modulated radiation therapy plan: by adding one or two segments to the two opposing tangential beams, hot spots receiving in excess of 105% are removed and homogeneous dose distribution is achieved.
Supraclavicular irradiation was prescribed to all the patients with involved lymph nodes. And it was also prescribed to the patients with T2 tumors who had lymphovascular invasion or high grade histology according to the physician's decision. For patients having a medially located tumor with axillary lymph nodal involvements, an internal mammary node chain was irradiated according to the physician's decision. A tumor bed was identified as the scar and surgical clips with a 2-cm margin. Boost was administered using an electron beam to a dose of 10 Gy and electron beam energy was selected according to the depth of the tumor bed.
4. Patient evaluation
Acute toxicities were scored and recorded weekly utilizing the Radiation Therapy Oncology Group acute radiation morbidity scoring criteria during treatment and 1 month after radiotherapy [16]. Then patients were followed up on every 3 to 4 months during the first year, then every 6 to 12 months thereafter. Late toxicities including fibrosis, fat necrosis, and telangiectasia were assessed at each visit and scored following the Common Terminology Criteria for Adverse Events ver. 4.0. Photos of the breast were taken at the initial visit, radiation therapy completion day, 2 years after RT, and 5 years after RT. Using these photos, the cosmetic outcome was retrospectively scored as "Excellent", "Good", "Fair" or "Poor" according to the Harvard scale by a radiation oncologist [17].
5. Statistical analysis
The aim of this study was to see local control, disease-free survival, and overall survival as well as acute and late toxicity and cosmetic outcome. The Kaplan-Meier method was used to estimate local control, disease-free survival, and overall survival. The log-rank test was used to perform univariate analysis and the Cox regression analysis was used to perform multivariate analysis on selected potential prognostic factors.
Results
1. Characteristics and clinical outcomes
Tables 1 and 2 list the patient and treatment characteristics. The human epidermal receptor type 2 (HER2) status is not described in Table 1 because the HER2 status was determined in only 4 patients by methods of in situ hybridization. The median follow-up duration was 7.3 years (range, 2.4 to 11.7 years). Ipsilateral breast recurrence was observed in 3 of the 214 patients. None of them had regional recurrence nor distant metastasis. The 5- and 10-year local control rates were 99.1% and 97.8%, respectively. A total of 3 regional recurrences were diagnosed. One patient also had distant metastasis. Two were in the supraclavicular fossa and 1 in the internal mammary area. Fifteen patients developed distant metastasis. The disease-free survival rate was 92.1% at 5 years and 82.7% at 10 years, and the overall survival rate was 99.5% and 96.4% at 5 and 10 years, respectively (Fig. 2).
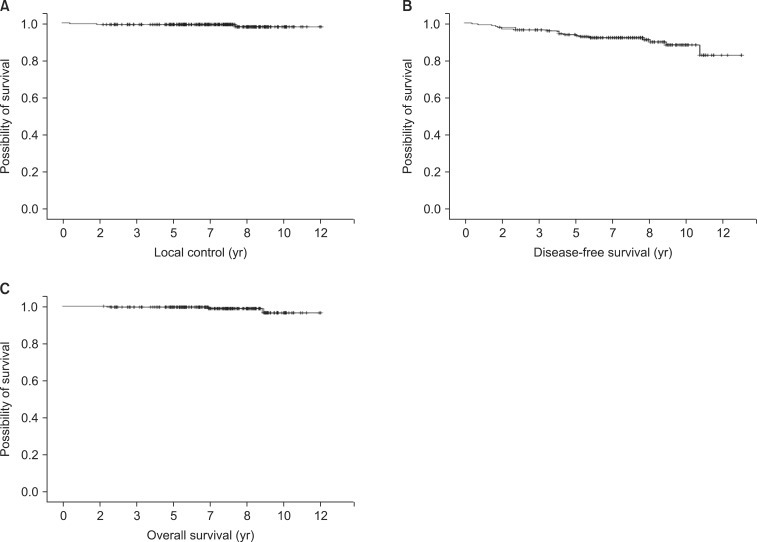
Kaplan-Meier survival analysis: local control (A), disease-free survival (B), and overall survival (C) of patients with early-stage breast cancer treated with forward intensity-modulated radiation therapy (n = 214).
Table 3 summarizes the univariate analysis of factors associated with local control, disease-free survival, and overall survival. Tumor size (p = 0.03), pathologic grade (p = 0.005), and N stage were significantly associated with the disease-free survival and the pathologic grade was the only factor significantly associated with the overall survival. The multivariate analysis identified the pathologic grade and N stage as independent factors for the disease-free survival (Table 4).

Results of univariate analyses of factors affecting local control (LC), disease-free survival (DFS), and overall survival (OS)
2. Toxicity and cosmetic outcome
The majority of patients had grade 0 or 1 acute toxicity (82.2%). There was grade 3 skin desquamation in two patients and no grade 4 acute toxicity was observed. The information about late toxicity was available in 189 patients. Only 15 patients (7.9%) experienced grade 2 late toxicity. More specifically, there were 11 cases of lymphedema with marked discoloration or leathery skin texture of the ipsilateral arms, 3 cases of subcutaneous fibrosis and 1 case of fat necrosis.
In 169 of 189 patients (89.4%), the treated breast was nearly identical to the untreated breast or slightly different than the untreated breast. These patients' cosmetic outcomes were scored as excellent or good following the Harvard scale.
Discussion and Conclusion
In this single institutional retrospective study, the forward IMRT technique used in our clinic showed excellent local control and a low rate of clinical toxicities as well as good cosmetic outcomes.
In breast RT, the IMRT technique was explored to achieve a homogeneous dose distribution as well as to improve the toxicity and cosmetic results. Indeed, several randomized trials have demonstrated the reduction in clinically observed late changes and better cosmesis in patients treated with IMRT [12-15,18,19].
In 2000, we performed a dosimetric analysis comparing the wedge and forward IMRT plan and concluded that a more favorable dose distribution could be obtained with a forward IMRT plan. Fig. 3 shows a case of improved dose homogeneity by using forward IMRT. Despite the analysis not being performed prospectively or published, based on our experiences, we routinely have treated the breast cancer patients with a forward IMRT technique since 2001. Recently Danish Breast Cancer Cooperative Group reported that a dose distribution in the CTV breast of 95% to 107% (normofractionated radiation therapy) is acceptable according to the International Commission on Radiation Units and Measurements criteria [20]. Treating the all patients using the forward IMRT technique can be criticized in terms of cost-effectiveness. However, in order to obtain even better cosmetic outcome, we have used the forward IMRT technique and the burden was affordable level in our clinic.
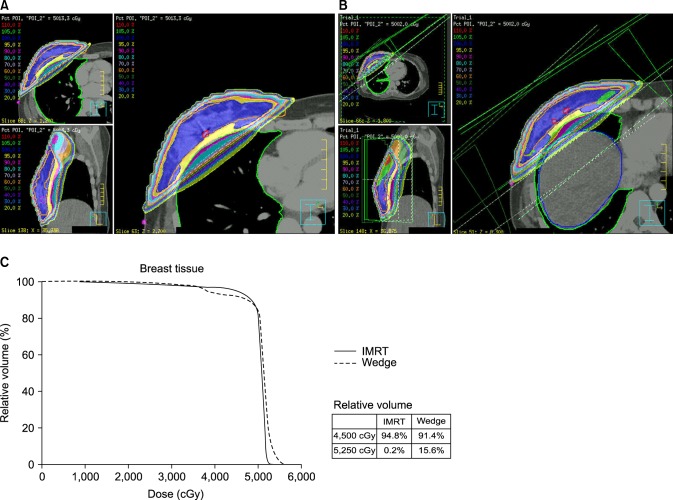
Dose distribution of forward intensity-modulated radiation therapy (IMRT) plan (A) and two tangential field plans using wedges (B) for same patient. The dose volume histogram (DVH) for breast tissue is shown (C). These dose distribution and DVHs show that by using the IMRT plan, more breast volume received above 95% of the prescribed dose at the same time the relative breast volume was receiving more than 105% of the prescribed dose (5,000 cGy), which is reduced compared to the wedge plan.
Whereas, benefits on local control should be limited as a breast target volume especially lumpectomy bed could be sufficiently covered by the conventional wedge-based radiation therapy techniques. Furthermore, the breast IMRT technique has the potential risk of compromised local control secondary to the use of several segments. The breathing motion during the treatment can induce intrafractional breast motion, which can countervail the effect of a more delicate treatment technique. In 2004, Frazier et al. [21] applied the gated CT using the active breath control device to create scans of the breast at normal respiration, and they figured out that dose distributions were fairly insensitive to breast motion during normal respiration.
The aim of this study was to observe long-term local control in patients treated with breast forward-IMRT and the local control rate was 99.1% at 5 years and 97.8% at 10 years. This result is not inferior to that of conventional radiation therapy and even higher than previously reported results in other trials using IMRT techniques.
There has been only one study observing long-term local control of breast IMRT. McDonald et al. [15] reported the outcomes of 240 patients with early-stage breast cancer in which 121 patients were treated with forward-planned breast IMRT versus 119 patients with conventional radiation therapy. They also reported the similar result to this study with excellent local control and significantly decreased clinical toxicities.
In addition to the excellent local control, this study showed a low rate of acute and late toxicity. Only two patients experienced grade 3 acute skin desquamation. A total of 15 patients (7.4%) had grade 2 late toxicities, which were mostly lymphedema followed by subcutaneous fibrosis and fat necrosis. All the patients who developed grade 2 lymphedema had been treated with axillary lymph node dissection except one patient. We presume that the lymphedema of these patients are more related to the extent of lymph node dissection rather than the technique of radiation therapy.
Limitations existed in the evaluation of the cosmetic outcome. When considering the photographs before radiation therapy, most patients who scored as "fair" or "poor" already had significant asymmetry in their breasts' appearance. It can be definitely concluded that the cosmetic outcome is closely related to the surgical technique. To distinguish the impact of the surgical technique on cosmesis, the shape of the treated breast should be compared with the appearance of the unilateral breast after surgery and the degree of each difference should be measured and graded. However, there are no reliable tools to measure in this way so far.
The results from this study are constrained by all the inherent flaws and biases of a retrospective study. We analyzed the patients treated during long periods, about 8 years. The method of treatment has changed during this period. For example, HER2 fluorescence in situ hybridization analysis and Herceptin were not used for the patients treated earlier. Inhomogeneity of the treatment characteristics could affect the treatment outcomes. Another limitation of this study was in the evaluation of the cosmetic outcome as shown before.
In summary, the whole breast radiation therapy as adjuvant treatment using the forward IMRT technique showed excellent long-term local control as well as favorable outcomes of toxicity and cosmesis.
Notes
No potential conflict of interest relevant to this article was reported.