Whole pelvic intensity-modulated radiotherapy for high-risk prostate cancer: a preliminary report
Article information
Abstract
Purpose
To assess the clinical efficacy and toxicity of whole pelvic intensity-modulated radiotherapy (WP-IMRT) for high-risk prostate cancer.
Materials and Methods
Patients with high-risk prostate cancer treated between 2008 and 2013 were reviewed. The study included patients who had undergone WP-IMRT with image guidance using electronic portal imaging devices and/or cone-beam computed tomography. The endorectal balloon was used in 93% of patients. Patients received either 46 Gy to the whole pelvis plus a boost of up to 76 Gy to the prostate in 2 Gy daily fractions, or 44 Gy to the whole pelvis plus a boost of up to 72.6 Gy to the prostate in 2.2 Gy fractions.
Results
The study cohort included 70 patients, of whom 55 (78%) had a Gleason score of 8 to 10 and 50 (71%) had a prostate-specific antigen level > 20 ng/mL. The androgen deprivation therapy was combined in 62 patients. The biochemical failure-free survival rate was 86.7% at 2 years. Acute any grade gastrointestinal (GI) and genitourinary (GU) toxicity rates were 47% and 73%, respectively. The actuarial rate of late grade 2 or worse toxicity at 2 years was 12.9% for GI, and 5.7% for GU with no late grade 4 toxicity.
Conclusion
WP-IMRT was well tolerated with no severe acute or late toxicities, resulting in at least similar biochemical control to that of the historic control group with a small field. The long-term efficacy and toxicity will be assessed in the future, and a prospective randomized trial is needed to verify these findings.
Introduction
The appropriate extent of the radiation field remains controversial, especially in patients with high-risk prostate cancer. The probability of lymph node metastasis is considerable even in patients with negative imaging studies [1,2]. Therefore, the effect of extended field radiotherapy (RT), which includes the whole pelvis, was tested in large prospective trials in high-risk patients. Comparisons of the efficacy of whole pelvic RT (WPRT) to that of prostate-only RT (PORT) showed that the long-term progression-free survival rates were similar in both arms [3-5]. However, these studies were designed before the publication of dose-escalation studies [6-9], and the pelvic radiation field was insufficient. In the dose-escalation era, the superiority of WPRT over PORT was confirmed in a large retrospective study in which the application of 75.6 Gy to the prostate resulted in a better biochemical disease control rate in the WPRT group than in patients receiving PORT [10].
The use of WPRT consequently raised concerns regarding the increased incidence of radiation-related toxicities. Acute gastrointestinal (GI) and genitourinary (GU) complications were more frequently reported in patients treated with WPRT than in those receiving PORT [11,12]. Regarding late complications, small but insignificant increases were noted in most series [6,9,10,13]. Dosimetric studies revealed that intensity-modulated radiotherapy (IMRT) significantly reduced the volume of the bladder and rectum irradiated with high doses in men receiving WPRT [14-18]. Accordingly, acute GI [17] and acute GU [18] toxicities were reduced with the use of IMRT.
In our institution, high-risk prostate cancer patients were treated with dose escalated WPRT. IMRT, endorectal balloon (ERB), and image guidance were used to reduce acute and late toxicities. The primary endpoint of the present study was to assess the clinical efficacy of WPRT in terms of biochemical failure-free survival. The secondary endpoint was to evaluate radiation-related toxicities.
Materials and Methods
The treatment records of biopsy proven prostate adenocarcinoma patients treated with definitive aim between 2008 and 2013 were reviewed. A total of 83 consecutive, high-risk patients received whole pelvic IMRT (WP-IMRT). Of these, 13 were excluded for the following reasons: follow-up period <6 months (n = 10), Roach score <15% (n = 2), or lost to follow-up (n = 1). Of the remaining 70 patients, those meeting any of the following criteria were included in the analysis: clinical T3a stage, or Gleason score (GS) 8-10, or initial prostate-specific antigen (PSA) level >20 ng/mL. These are high-risk factors for recurrence according to the National Comprehensive Cancer Network guidelines [19]. Although high risk, two patients with Roach score <15% were excluded. The pretreatment evaluation consisted of a complete medical history, magnetic resonance imaging (MRI), bone scan, and laboratory tests including PSA.
Computed tomography (CT) simulation was performed with the patients in the supine position with ankle immobilization. The region from the second lumbar vertebrae to the proximal one-third of the femur was scanned with a slice thickness of 2.5 mm. Patients were instructed to empty their bowel and bladder immediately before simulation and subsequent treatment sessions. To reduce the volume of rectum irradiated, and to fix the prostate effectively, the ERB was used for all patients starting in 2009. The details of the balloon and procedure were described previously by D'Amico et al. [20]. Briefly, a homemade rectal balloon was inserted and inflated with 60 mL of air. The inflated balloon had a diameter of approximately 40 mm and a length of approximately 60 mm. The gross target volume (GTV) included the whole prostate and the involved lymph nodes. The clinical target volume (CTV) included the GTV, seminal vesicles, and internal iliac, external iliac, and obturator nodal regions. The upper limit of the CTV was the level of the common iliac bifurcation, which was generally located at or just above the L5/S1 junction. The planning target volume was a 5 to 7 mm expansion of the CTV. After pelvic RT, the boost treatment included only the GTV, seminal vesicles, and metastatic lymph nodes. Cone-beam CT (CBCT) for image guidance was performed daily after 2011 to improve the setup stability and ERB localization. CBCT imaging results were examined daily by a physician, focusing on GTV volume and the anterior rectal wall. All patients were treated using WP-IMRT. Doses and fractionation schemes were modified during the study period as follows: before 2011, the whole pelvis and the boost doses were 46 Gy and 76 Gy, respectively, administered in 2 Gy fractions. After 2011, the fraction size was increased to 2.2 Gy, and the whole pelvis and boost doses were 44 Gy and 72.6 Gy, respectively. IMRT schemes using five to seven fields were created using Eclipse 10.0 (Varian Medical Systems, Palo Alto, CA, USA). Androgen deprivation therapy (ADT) was administered to the majority of the patients at the discretion of the referring urologists.
PSA level was assessed within 3 months after RT, every 3 months for the next 2 years, and every 6 months thereafter. Biochemical failure was defined as an increase in the PSA level of 2 ng/mL or more above the PSA nadir after RT, according to the Phoenix definition [21]. Local recurrence was defined as disease recurrence detected in imaging studies, including CT or MRI. The patients were examined weekly during the course of treatment. Acute complications were those occurring within 3 months after treatment and late complications were those occurring after 3 months of treatment. Toxicities were recorded using the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.02, with the addition of the toxicity criteria of the Radiation Therapy Oncology Group (RTOG) morbidity grading scale.
Survival and the incidence of late toxicity were analyzed using the Kaplan-Meier method. Acute toxicity was expressed as a crude rate. The univariate prognostic factor analysis performed using the log-rank test included the following variables: patient age, T & N stage, GS, pretreatment PSA, PSA nadir, and the use of hormonal therapy. All statistical analyses were performed using SPSS ver. 21 software (IBM, Armonk, NY, USA).
Results
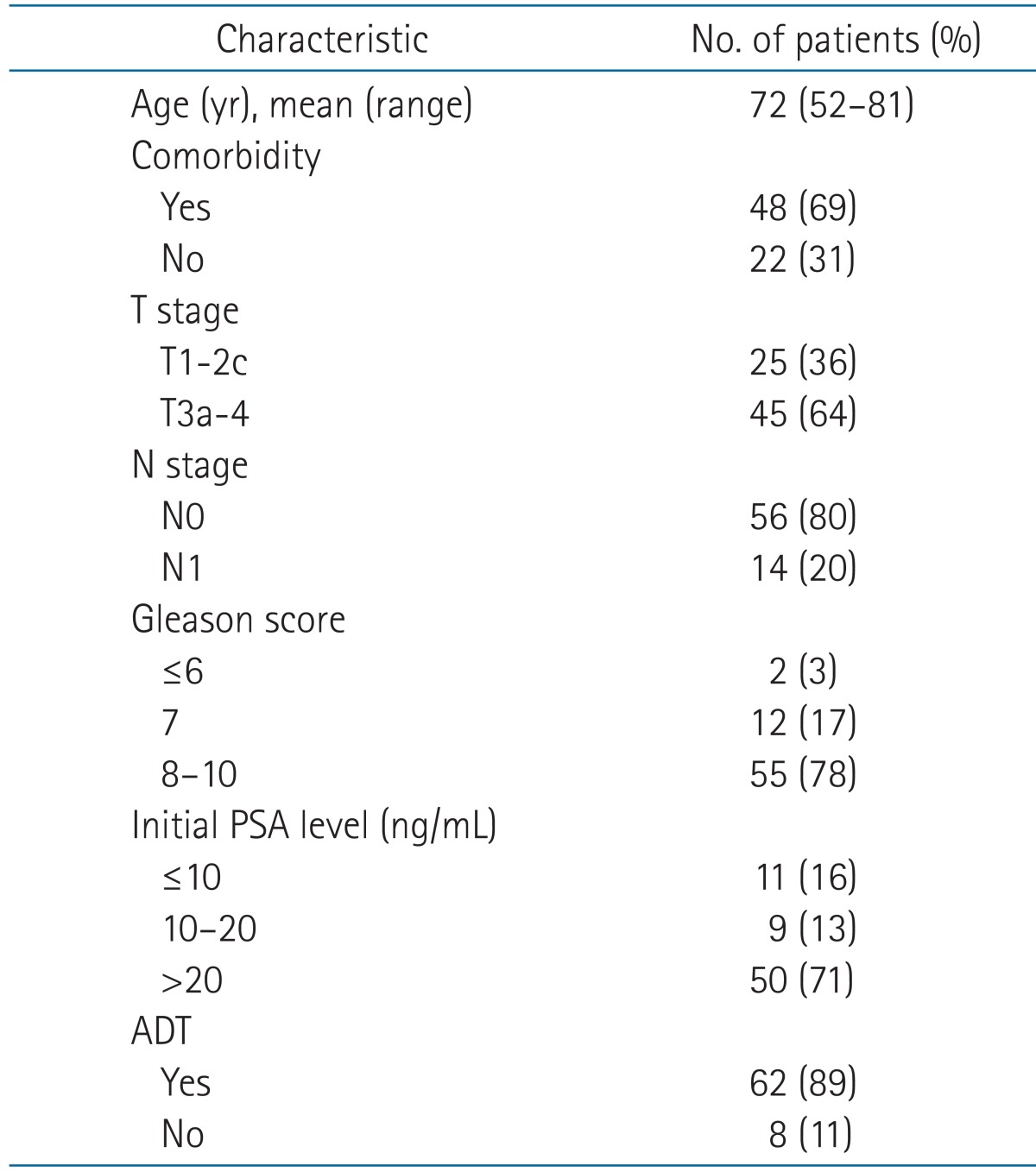
The characteristics of the 70 patients analyzed are listed in Table 1. Of the 70 patients in the study cohort, 51 received up to 72.6 Gy in daily fractions of 2.2 Gy to the prostate and 13 received up to 76 Gy in daily fractions of 2 Gy to the prostate. In the remaining six patients, the whole pelvic doses were 46 Gy, but the prostate doses were heterogeneous, ranging from 70 to 80.5 Gy. ADT was administered in 62 patients in the following sequences: neoadjuvant + concurrent + adjuvant (n = 39), concurrent + adjuvant (n = 16), neoadjuvant (n = 5), concurrent (n = 1), and adjuvant (n = 1). The median duration of hormonal therapy was 16 months (range, 5 to 60 months). Total androgen blockade was used in 49 patients and gonadotropin-releasing hormone agonist alone in 13.
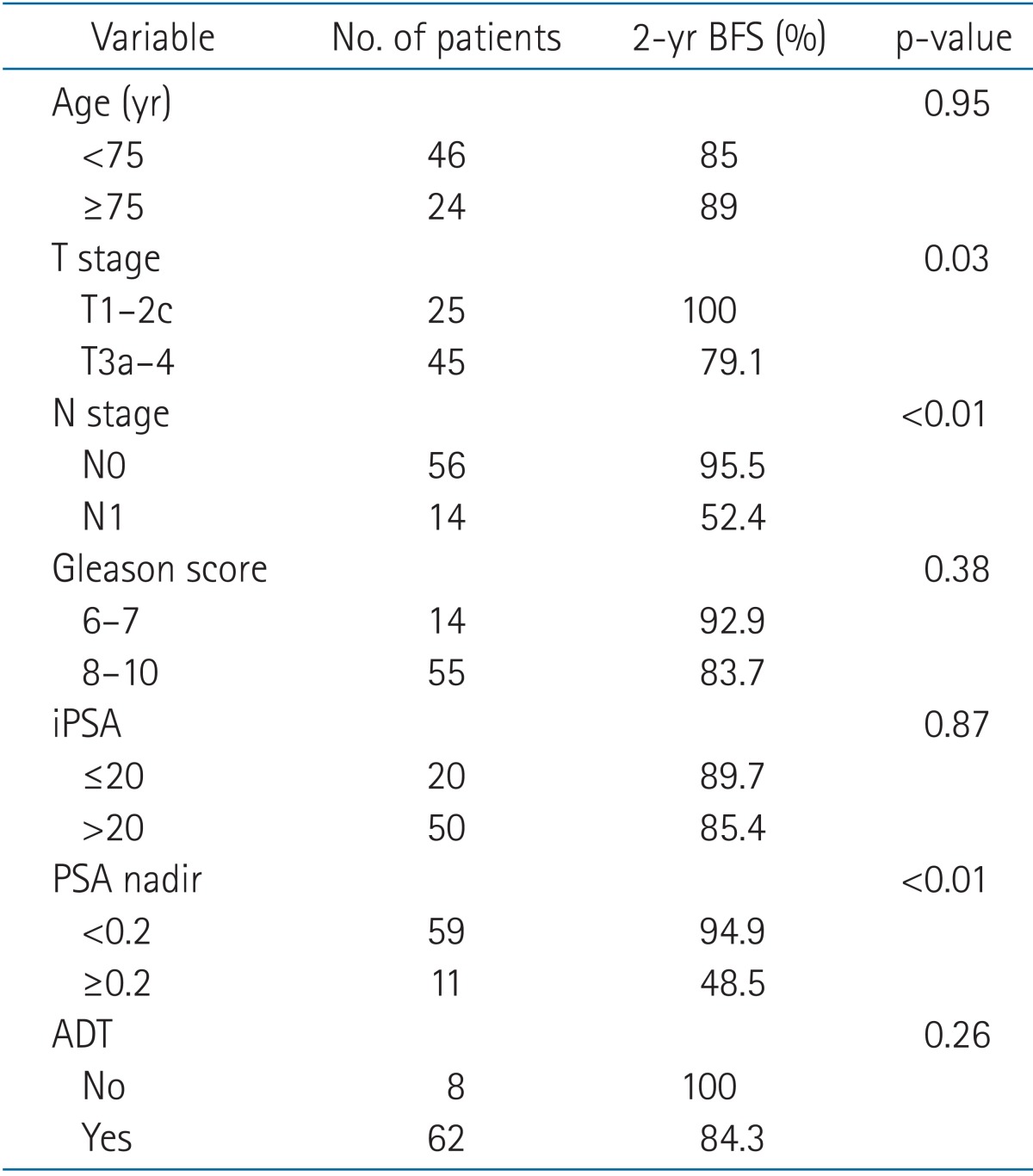
The median follow-up duration was 19 months (range, 2 to 61 months). At 2 years, the biochemical failure-free survival, local recurrence-free survival, and distant metastasis-free survival rates were 86.7%, 93.0%, and 92.9%, respectively. The disease-free survival and overall survival rates were 87.6% and 91.8%, respectively. In the prognostic analysis for biochemical failure-free survival, the T/N stage and PSA nadir had p-values ≤ 0.05. The results of the prognostic factor analysis are summarized in Table 2.
The numbers of patients with acute and late toxicities and their grades are listed in Table 3. RT was well tolerated with no severe toxicity. Acute GI and GU toxicities of any grades were observed in 47% and 73% of patients. None of the patients experienced grade 3 acute GI toxicity. The symptoms of GI toxicity were anorexia (n = 10), abdominal pain (n = 9), dyspepsia (n = 8), diarrhea (n = 7), rectal discomfort (n = 5), abdominal distension (n = 3), and nausea (n = 1). The only grade 2 symptom was diarrhea requiring medication. The only acute grade 3 GU toxicity was an increase in urinary frequency, with voiding intervals of less than 1 hour. Medication was needed until the completion of RT. Other grade 1-2 acute urinary symptoms included nocturia (n = 30), increased urinary frequency (n = 21), dysuria (n = 20), urgency (n = 13), incontinence (n = 3), and hematuria (n = 2). Overall, the incidence rates of late GI and GU toxicity of any grade were 16% and 30%, respectively. Regarding late GI toxicity, grade 3 radiation proctitis was diagnosed in three patients who showed repeated blood tinged stool requiring transfusion (n = 2) or argon plasma coagulation (n = 1). Regarding late GU toxicity, the only grade 3 toxicity was gross hematuria. Cystoscopic evaluation showed radiation induced mucosal changes. The rate of late grade 2 or worse toxicity at 2 years was 12.9% for GI and 5.7% for GU. None of the patients experienced late grade 4 toxicity.
Discussion and Conclusion
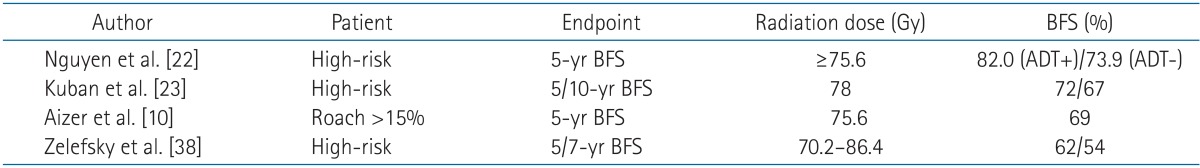
The present study was a preliminary analysis of the effects of WP-IMRT in high-risk prostate cancer patients. The results showed acceptable outcomes in terms of biochemical control and toxicity profiles, although further studies with long-term follow-up are necessary to verify these results. The results of previous studies using PORT in high-risk patients are summarized in Table 4. A study from the MD Anderson Cancer Center showed a 5-year biochemical failure-free survival of 82% in patients treated with 75.6 Gy plus ADT for ≥ 2 years [22]. Other trials that included patients treated with higher radiation doses (>75 Gy) [10,23] showed 5-year biochemical control rates of approximately 70%. In the present study, the biochemical control rate in high-risk patients was 86.7% at 2 years. Considering that the present study included a higher proportion of patients with stage T3 prostate cancer, GS 8-10 and PSA >20 ng/mL than previous studies [10,22,23], our results can be interpreted as at least comparable or better than those of previously published studies. However, the present work is a preliminary report with short-term follow-up, and drawing definitive conclusions is therefore difficult. Nevertheless, the patient outcomes in the present study were comparable to those of previous studies possibly because of the use of an adequate radiation field and the frequent use of combined hormonal therapy. The relation between elective pelvic node irradiation and PSA relapse is controversial. In the RTOG 9413 trial, WPRT showed a statistically significant benefit over PORT, as demonstrated in the 4-year progression-free survival (54% vs. 47%, p = 0.02); however, a significant difference was no longer observed in the long-term update [3,4]. In another prospective study, GETUG-01, prostate cancer patients were randomized to WPRT or PORT treatment groups, and no differences in 5-year progression-free survival were observed (66% in WPRT vs. 65% in PORT, p = 0.34). Because the definition of progression in the RTOG 9413 trial included death from any cause, non-prostate cancer-related deaths may have dominated over prostate cancer-related events in the long-term follow-up. In the GEUTG-01 study, more than 50% of the patients had a risk of nodal involvement of <15% and the upper limit of the radiation field was relatively low (S1-2). Furthermore, these trials were designed before the publication of dose-escalation studies: in RTOG 9413, the prescribed doses to the pelvis and prostate were 50.4 Gy and 70.2 Gy, respectively, and in GETUG-01, they were 46 Gy and 66 to 70 Gy, respectively. High-dose RT, which is currently in use, was tested in a comparative setting in a retrospective study conducted at Yale University in which the effects of WPRT and PORT were compared in patients with a Roach score >15%. The prescribed dose in this study was 75.6 Gy in 1.8 Gy fractions. When the α/β ratio of a prostate tumor is assumed to be 2, the calculated equivalent dose in 2 Gy fractions (EQD2) is 71.8 Gy. In the Yale study, the four-field box technique was used for the treatment of the whole pelvis and the 4-year biochemical failure-free survival rates for patients receiving WPRT and PORT were 86% and 69%, respectively (p < 0.01) [10]. In the present study, the irradiated dose was changed from 76 Gy in 2 Gy fractions to 72.6 Gy in 2.2 Gy fractions (EQD2 = 76.2 Gy), and IMRT was used in all treatment plans. In addition, the follow-up period was shorter than that of the Yale study. Otherwise, the patient population and treatment regimens were similar to those of the whole pelvis arm in the Yale study, and both studies had similar outcomes. Although we did not include a control group and longer follow-up is needed, the biochemical control in the present study was comparable to that reported in previous studies. However, the differences in the follow-up duration make a direct comparison difficult. Our results suggest that patients with high-risk prostate cancer in whom the predicted risk of nodal involvement exceeds 15% may benefit from elective pelvic nodal irradiation in terms of biochemical control. However, additional studies with longer follow-up are necessary to draw definitive conclusions.
During the treatment period, only one patient experienced grade 2 GI toxicity (diarrhea), and no grade 3 toxicities were reported. Acute grade 2 and grade 3 GU toxicities were reported in 15 patients and one patient, respectively. Anorexia and nocturia were the most frequent symptoms and they resolved spontaneously during follow-up. An association between IMRT and a reduction in the incidence of acute complications has been reported previously. In a study conducted at Memorial Sloan-Kettering Cancer Center, Ashman et al. [17] compared dosimetric outcomes and toxicities in 13 patients treated with two-dimensional, three-dimensional conventional RT (3D-CRT), and IMRT and showed that the bowel volume receiving more than 45 Gy (V45), the mean bowel dose, rectal V45, and bladder V45 were all significantly reduced with IMRT. The acute GI and GU toxicities were minimal with no case of grade 3 or higher toxicity. In the present study, the rate of late grade 2 or worse toxicity at 2 years was 12.9% for GI and 5.7% for GU toxicities, which is a better result than that of the RTOG 9413 whole-pelvic group, which used lower radiation doses administered using the 4-field technique. In that study, the rate of 5-year GI and GU toxicities ≥ grade 2 were 15.2% and 14.9%, respectively [24]. Quon et al. [25] analyzed the toxicity rates of dose escalated WPRT using a whole-pelvic dose of 45 Gy in 1.8 Gy fractions and a concomitant 22.5 Gy prostate IMRT boost. The 4-year GI and GU toxicities ≥ grade 2 were 5.8% and 10.1%, respectively. Compared to the data from our institution, the EQD2 was lower for the pelvis and higher for the prostate boost [25], which may have contributed to the higher GI and lower GU late toxicities in the present study. An association between the prostate dose and chronic GU toxicity has been suggested in previous studies [26,27]. The reported late GI and GU toxicity rates in dose escalated PORT studies are 5% to 46% and 11% to 39%, respectively [28-31]. Generally, most late rectal toxicities attributed to RT appear 12 to 18 months after completion of treatment. However, this issue is controversial. In the Medical Research Council RT01 trial, patients were treated with 64 to 74 Gy and the 2- and 5-year cumulative proportion of patients showing treatment-related toxicities was reported. Grade 2 or higher GI toxicity rates at 2 and 5 years were 14% and 24%, respectively. Although bowel function complications developed mostly within 2 years, with a prevalence of 6 to 24 months, other GI and GU complications increased after 2 years. The GU toxicity rates at 2 and 5 years were 6% and 8%, respectively [31]. The later onset of late GU toxicity compared to GI toxicity was analyzed in several studies. Zelefsky et al. [26] used a dose of 66 to 81 Gy and a median follow-up of 10 years and showed that the median time to development of GU symptoms of grade ≥ 2 was 30 months, compared with 17 months for patients with GI side effects of grade ≥ 2. Gardner et al. [32] used 77.4 Gy and showed that the actuarial risk of grade ≥ 2 hematuria was 21% at 5 years and 47% at 15 years; the incidence of GU toxicities increased progressively during the entire study period, which had a median follow-up duration of 13.1 years. Therefore, longer follow-up may not worsen our GI complication results, whereas further follow-up for late GU complications will be needed considering the different time trends of late GI and GU toxicities. IMRT was shown to lower the rate of late toxicities in high-dose prostate RT. Zelefsky et al. [26] compared the long-term tolerance of 3D-CRT and IMRT and showed that IMRT reduced the rate of GI toxicity ≥ grade 2 by 8% (13% to 5%, p < 0.01) at the 10-year follow-up. The rate of 10-year GU toxicity ≥ grade 2 in patients treated with IMRT was 17% [33]. The prostate gland immobilization and rectal sparing effects of the ERB were tested by several investigators [34]. When combined with IMRT, it reduced prostate motion and had a favorable rectal toxicity profile [35,36]. Zelefsky et al. [37] showed the benefits of the direct visualization of the pelvic anatomy. In the present study, we used CBCT in all treatments for proper setup and rectal sparing.
The present study had several limitations. Despite the large number of patients included, the study was limited by its retrospective design, the heterogeneous use of ADT and a short follow-up period. Therefore, the authors will assess the long-term efficacy and toxicity again in the future to verify the benefits of WP-IMRT for the treatment of high-risk prostate cancer patients.
In conclusion, WP-IMRT was well tolerated with no severe acute or late toxicities. The present preliminary report shows that WP-IMRT results in acceptable biochemical control rates compared to those reported previously with a small field. The long-term efficacy and toxicity will be assessed again in the future, and a prospective randomized trial is needed to verify the present findings.
Notes
No potential conflict of interest relevant to this article was reported.