 |
 |
AbstractPurposeWe evaluated the effectiveness and safety of ipsilateral radiotherapy for the patient with well lateralized tonsil cancer: not cross midline and <1 cm of tumor invasion into the soft palate or base of tongue.
Materials and MethodsFrom 2003 to 2011, twenty patients with well lateralized tonsil cancer underwent ipsilateral radiotherapy. Nineteen patients had T1-T2 tumors, and one patient had T3 tumor; twelve patients had N0-N2a disease and eight patients had N2b disease. Primary surgery followed by radiotherapy was performed in fourteen patients: four of these patients received chemotherapy. Four patients underwent induction chemotherapy followed by concurrent chemoradiotherapy (CCRT). The remaining two patients received induction chemotherapy followed by radiotherapy and definitive CCRT, respectively. No patient underwent radiotherapy alone. We analyzed the pattern of failure and complications.
ResultsThe median follow-up time was 64 months (range, 11 to 106 months) for surviving patients. One patient had local failure at tumor bed. There was no regional failure in contralateral neck, even in N2b disease. At five-year, local progression-free survival, distant metastasis-free survival, and progression-free survival rates were 95%, 100%, and 95%, respectively. One patient with treatment failure died, and the five-year overall survival rate was 95%. Radiation Therapy Oncology Group grade 2 xerostomia was found in one patient at least 6 months after the completion of radiotherapy.
IntroductionIn several pathologic studies for tonsil cancer, the rate of occult lymph nodes metastasis in the contralateral neck has been reported about 15%, and this rate is significantly higher in T3 and T4, up to 20%-30% [1,2]. For the patient with tonsil cancer, conventional radiotherapy (RT) has routinely encompassed the bilateral neck, using parallel opposite bilateral ports [3]. In this technique, salivary glands on both sides cannot be spared, so xerostomia is a major complication of RT for tonsil cancer. With the development of a precise RT technique, it is possible to spare at least one parotid gland. Limited RT to ipsilateral neck is another option for sparing salivary glands. Ipsilateral RT can spare the parotid and submandibular glands on the contralateral side, and this can contribute to less severe treatment-related xerostomia [4-6].
The concern about regional failure in the contralateral neck might be a major hurdle of ipsilateral RT. On the contrary, Perez et al. [7] reported that well lateralized tonsil cancer has a low incidence of contralateral neck metastasis. Generally, less than 1 cm of medial extension to the soft palate or to base of tongue (BOT) is accepted as well-lateralized tonsil cancer [8,9]. O'Sullivan et al. [9] reported a long-term result of ipsilateral RT in tonsil cancer, and the rate of opposite neck failure was very low, 3.5%. Jackson et al. [10] reported a similar failure rate of 2.6% in the contralateral neck for patients with tonsil cancer receiving ipsilateral RT. Thereby ipsilateral RT has been adopted as a practice in selected patients with well lateralized tonsil cancer.
In our institution, selected patients with well lateralized tonsil cancer have received ipsilateral RT since 2003, using three-dimensional radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). The purpose of this study was to evaluate the effectiveness and safety of ipsilateral RT for patients with well lateralized tonsil cancer, by analyzing the patterns of failure and complications.
Materials and MethodsA total of 25 patients with tonsil cancer underwent ipsilateral RT from 2003 to 2011 at the Department of Radiation Oncology, Seoul National University Hospital. Of these patients, 5 patients who had T4 or N3 disease were excluded in this study, because ipsilateral RT was performed as palliative aim. Decision of ipsilateral RT or bilateral RT was made by following guidelines: not cross midline and <1 cm of tumor invasion into the soft palate or BOT. For pretreatment imaging work up, neck computed tomography (n = 18) or magnetic resonance imaging (n = 11) was performed in all patients, and positron emission tomography using fluorine-18 2-deoxy-D-glucose tracer was obtained in 14 patients. Medical records were reviewed with the approval of the Institutional Review Board (1108-139-376). Nineteen patients had well lateralized T1 or T2 tumors, whereas one patient had T3 tumor. None of patients had neck node metastasis in the opposite site of primary tumor, and eight patients (40%) had N2b disease. Patient characteristics are listed in Table 1.
1. RadiotherapyFourteen patients (70%) received 3D-CRT and six patients (30%) received IMRT. Definitions of clinical target volume (CTV) were as follows: primary tumor or tumor bed with 5-mm margin as CTV1, the level of involved neck node and retropharyngeal node as CTV2, and next echelon of involved neck node level as CTV3. Lower neck nodes (level IV) were treated in 12 patients. Especially, all patients with N2b received lower neck irradiation. The XiO RT planning system (Elekta CMS, Stockholm, Sweden) was used for 3D-CRT. In IMRT, the Eclipse system (Varian Medical Systems, Palo Alto, CA, USA) was used for inverse treatment planning. Planning target volume (PTV) had a 3-mm margin from CTV being similarly defined as in 3D-CRT.
We prescribed a dose to CTVs in 3D-CRT and PTVs in IMRT, for delivery of more than 97% of the prescribed dose to 97% of the target volumes [11]. The prescribed dose to CTV1/PTV1 was, for patients with definitive RT, median 70 Gy (range, 66 to 70 Gy) in 3D-CRT with the daily dose of 2Gy and 67.5 Gy in IMRT with the daily dose of 2.25 Gy (Table 2). For patients with postoperative RT, median 66 Gy (range, 66 to 70 Gy) in 3D-CRT with the daily dose of 2 Gy and 63 Gy in IMRT with the daily dose of 2.25 Gy were prescribed to CTV1/PTV1. Three to four fields of 6 MV photon beams were used in 3D-CRT, and five to six fields of 6 MV photon beams were used in IMRT. The following criteria were used for organs at risk: <20 Gy to half of the parotid gland, <54 Gy to the brain stem, <45 Gy to the spinal cord, and <50 Gy to the optic chiasm and the optic nerve.
2. SurgerySurgery followed by RT was performed in fourteen patients (70%): 3 received extended tonsillectomy, 5 received neck dissection, and 6 received extended tonsillectomy and neck dissection. Diagnostic tonsillectomy was not regarded as a radical surgery. Modified radical neck dissection was performed in 10 patients, and supraomohyoid dissection was done in 1 patients. Induction chemotherapy before surgery was given in two patients, and one of these patients underwent chemotherapy concomitantly with postoperative RT either. Two patients received concurrent chemoradiotherapy (CCRT) following surgery. Postoperative chemotherapy was considered when adverse feature exists, such as positive resection margin, extracapsular extension, or multiple lymph nodes involvement. Other ten patients underwent postoperative RT only.
3. ChemotherapyDefinitive RT was performed in 6 patients (30%): 1 underwent induction chemotherapy followed by RT, 1 had CCRT, and 4 received induction chemotherapy and CCRT. Three cycles of induction chemotherapy was given, and regimens were as follows. Docetaxel 70-75 mg/m2 (day 1) and cisplatin 75 mg/m2 (day 1) in every 3 weeks; paclitaxel 175 mg/m2 (day 1) and cisplatin 75 mg/m2 (day 1) in every 3 weeks; 5-fluorouracil (FU) 1,200 mg/m2 (day 1-4) and cisplatin 40 mg/m2 (day 1-2) in every 3 weeks; docetaxel 70 mg/m2 (day 1), cisplatin 40 mg/m2 (day 2-3), and 5-FU 1,200 mg/m2 (day 1-3) in every 3 weeks. During RT, cisplatin 30-35 mg/m2 (day 1) was delivered concurrently, in every week.
ResultsThe median follow-up time was 64 months (range, 11 to 106 months) for surviving patients. Of the twenty patients, 1 had local recurrences. At 5-year, local progression-free survival (LPFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) rates were 95%, 100%, and 95%, respectively. One patients with treatment failure died, and the 5-year overall survival (OS) rate was 95%.
1. Local failureThe patient with local recurrence had a pT2N2b disease: right tonsil mass and metastatic neck nodes in the right neck node level Ib and II. This patient underwent induction chemotherapy (docetaxel, cisplatin, and 5-FU) followed by right tonsillectomy and modified radical neck dissection. Thereafter this patient received CCRT with cisplatin: 70 Gy to CTV1 (tumor bed and right neck node level I) and 50 Gy to CTV2 (right neck node level II). With 3 months of follow-up time after the completion of RT, local recurrence occurred in the right tonsil (tumor bed) (Fig. 1). This patient expired about 1 month after the initiation of chemotherapy as a salvage treatment.
2. ComplicationDuring RT, six patients (30%) noted grade 3 mucositis: 4 received 3D-CRT and 2 received IMRT; 3 underwent primary surgery and 3 underwent definitive RT. Grade 2 xerostomia was found in one patient (5%), at least 6 months after the completion of RT. This patient with chronic xerostomia received induction chemotherapy and CCRT.
Discussion and ConclusionThe limited irradiation to the ipsilateral neck has been accepted as resulting in effective and favorable outcomes for well lateralized tonsil cancer [9,10]. However, there is no randomized control study about ipsilateral RT for tonsil cancer. Recently a prospective study of ipsilateral neck RT for node positive tonsil cancer published, while the median follow-up time was relatively short, 19 months [12]. In the present study, we report treatment outcomes of ipsilateral RT for tonsil cancer, with a long-term follow-up time.
O'Sullivan et al. [9] performed a pioneer study of ipsilateral RT for 228 patients with well lateralized tonsil cancer. A total of eight patients (3.5%) experienced opposite neck failure, and 38 patients (17%) experienced ipsilateral neck failure. Ipsilateral neck control was not as good as contralateral neck control. Particularly in seven patients with T4 disease, ipsilateral neck failure was seen in three patients (43%). Worse local control might result from a less precise RT technique; since the two-field wedged-pair technique was the standard, and CT planning was not used in this study. Also, the majority of patients received a relatively low dose of 50 Gy to primary lesion; this could be another reason for worse local control. Another pioneer study by Jackson et al. [10] reported the results of 178 patients who underwent ipsilateral RT for well lateralized tonsil cancer. Two or three wedged fields without target delineation were used, and the most common dose was 60 Gy in this study. Clear relationship of treatment results and T stage was manifested. Locoregional control rates were 91% in T1, 74% in T2, 51% in T3, and 53% in T4 patients. Local control rates showed similar results, 94% in T1, 79% in T2, 58% in T3, and 56% in T4. Authors suggested that more effective RT to deal with the primary disease could improve treatment results of ipsilateral RT.
In the present study, the five-year local and regional progression-free survival rates were 95% and 100%, respectively. The improvement of local and regional control, compared to past studies [3,9,10], was attributable to the development of precise RT techniques. In our study, most patients (76%) received 3D-CRT and others (24%) received IMRT. Several studies of ipsilateral RT for oropharyngeal cancer, using 3D-CRT or IMRT, showed similar outcomes as our study [12-15]. Both RT techniques can improve target coverage by tailoring isodose lines, thereby increasing locoregional control. Enhanced locoregional control might contribute better contralateral neck control and survival. Cerezo et al. [15] reported the results of ipsilateral RT using 3D-CRT for oral and oropharyngeal cancer patients. The total dose prescribed to the primary tumor was 66-70 Gy for definitive setting and 54-64 Gy for adjuvant setting. With a median follow-up time of 58 months, there was no locoregional recurrence. Furthermore, IMRT can provide improved target coverage compared to 2D-RT and 3D-CRT by employing an inverse treatment planning system and optimizing dose distribution in head and neck cancers [16-18]. IMRT can make a steep dose gradient between the PTV and the planning organ at risk volume. In the MD Anderson Cancer Center, 102 patients with tonsil cancer were treated with ipsilateral RT, and 67 patients underwent IMRT [14]. With the median follow-up time of 39 months, there was no recurrence in the primary site or ipsilateral neck.
Lim et al. [2] reported a pathologic result of contralateral elective neck dissection in tonsil cancer patients with clinically negative contralateral neck nodes. In this study, pathologically positive contralateral neck lymph nodes were found in 21% of patients with positive ipsilateral neck nodes (7 of 33). Six patients with contralateral occult metastasis had T3 or T4 disease. Authors suggested elective neck treatment in patients with tonsil cancer greater than T3. O'sullivan et al. [9] included 107 tonsil cancer patients with invasion into the palate or BOT. Most of contralateral neck failures (6 in 8 patients) occurred in these patients. Excluding these patients, contralateral neck failures were found in 2 patients. All of patients with contralateral neck failure had N1 disease. Authors recommended ipsilateral RT in patients with small amounts of medial extension (no more than 1 cm) to the palate or BOT. Neck nodes involvement was regarded to have a modest risk of contralateral neck failure. However, it should be noted that positive ipsilateral neck nodes patients had more extensive primary lesion.
In the contrast, Chronowski et al. [14] reported only 2 cases of contralateral neck failures in 102 patients with tonsil cancer treated with ipsilateral RT. In this study, none of the included patients had BOT invasion, while 22% of patients had N2b disease. Two patients with contralateral neck failure had T2N0 and TxN0 disease. In a prospective study of ipsilateral RT for tonsil cancer by Rusthoven et al. [12], there was no contralateral neck failure even though most of patients (16 of 20) had N2a or N2b disease. However this study included patients with no evidence of BOT or palate. Cerezo et al. [15] reported no contralateral nodal recurrence in patients with <1 cm of medial extension to the palate or BOT, while 10% and 25% of patients had T4 and N2a-b disease, respectively. In summary, ipsilateral RT showed favorable contralateral neck control even in tonsil cancer patients with positive neck nodes, when tumor invasion into the palate or BOT was not exist or confined less than 1 cm. In our study, similar with aforementioned studies, patients with extensive invasion into the palate or BOT were excluded, while patients with T3 or N2b disease were included. This could be a reason of excellent contralateral neck control of our study.
The major benefit of ipsilateral RT is to spare salivary glands on the opposite side. In our data, only one patient (4%) noted grade 2 xerostomia; others had grade 1 or lower xerostomia. This is a very favorable result, considering approximately 20%-30% rates of chronic xerostomia reported in prospective IMRT studies for oropharyngeal cancer [19,20]. Eisbruch et al. [21] compared salivary flow rates and scores of xerostomia-specific questionnaire (XQ) in the patients with head and neck cancer who underwent bilateral IMRT and ipsilateral RT. After RT, xerostomia improved over time, being attributed to salivary production from spared major salivary glands. The improvement was faster in patients with ipsilateral RT, while the difference narrowed at two years after RT. Saarilahti et al. [22] performed a study of 36 patients with head and neck cancer. All patients received IMRT sparing at least one parotid gland, and the contralateral SMG (cSMG) was spared in 16 patients. After 12 months of IMRT completion, unstimulated salivary flows were 60% and 25% of the baseline in patients in which the cSMG was spared and not spared (p = 0.006). Grade 2 or 3 xerostomia was less reported in patients with cSMG spared (p = 0.018).
About two-thirds of unstimulated salivary flow comes from the SMG, which produces mucinous saliva, contributing relief for subjective symptoms of xerostomia [23]. Generally, ipsilateral RT can spare the cSMG as well as the contralateral parotid gland, while bilateral RT may not sufficiently spare the cSMG when adjacent to target volumes. Additionally, improvement of other RT-related morbidity, such as dysphagia, could be expected in ipsilateral RT [4]. This can be explained by the volume effect of the upper aero-digestive tract which is involved in the swallowing process: to omit contralateral neck irradiation in ipsilateral RT rather than to use dose constraint in IMRT [24].
In conclusion, ipsilateral RT is a reasonable treatment option for well lateralized tonsil cancer. There was no regional failure in contralateral neck, even in N2b disease. A low rate of chronic xerostomia can be expected by sparing contralateral major salivary glands. Though our results with long-term follow-up time are promising, the limitations such as small number of patients and heterogeneous characteristics should be validated in a large randomized study.
AcknowledgmentsThis work was supported by a grant No. 04-2012-0440 from the SNUH Research Fund, a grant of the Korea Health technology R&D Project, Ministry of Health&Welfare, Republic of Korea (A111098 and A120313), and National R&D Program through the Dongnam Institute of Radiological & Medical Sciences (DIRAMS) funded by the Ministry of Education, Science and Technology (50595-2012).
References1. Olzowy B, Tsalemchuk Y, Schotten KJ, Reichel O, Harreus U. Frequency of bilateral cervical metastases in oropharyngeal squamous cell carcinoma: a retrospective analysis of 352 cases after bilateral neck dissection. Head Neck 2011;33:239–243, PMID: 20848445.
2. Lim YC, Lee SY, Lim JY, et al. Management of contralateral N0 neck in tonsillar squamous cell carcinoma. Laryngoscope 2005;115:1672–1675, PMID: 16148715.
3. Chang AR, Wu HG, Park CI, Kim KH, Sung MW, Heo DS. Retrospective analysis of the treatment results for patients with squamous cell carcinoma of tonsil. Cancer Res Treat 2005;37:92–97, PMID: 19956486.
4. Jensen K, Overgaard M, Grau C. Morbidity after ipsilateral radiotherapy for oropharyngeal cancer. Radiother Oncol 2007;85:90–97, PMID: 17604141.
5. Kagei K, Shirato H, Nishioka T, et al. Ipsilateral irradiation for carcinomas of tonsillar region and soft palate based on computed tomographic simulation. Radiother Oncol 2000;54:117–121, PMID: 10699473.
6. Corvo R, Foppiano F, Bacigalupo A, Berretta L, Benasso M, Vitale V. Contralateral parotid-sparing radiotherapy in patients with unilateral squamous cell carcinoma of the head and neck: technical methodology and preliminary results. Tumori 2004;90:66–72, PMID: 15143975.
7. Perez CA, Patel MM, Chao KS, et al. Carcinoma of the tonsillar fossa: prognostic factors and long-term therapy outcome. Int J Radiat Oncol Biol Phys 1998;42:1077–1084, PMID: 9869232.
8. Yeung AR, Garg MK, Lawson J, et al. ACR Appropriateness Criteria® ipsilateral radiation for squamous cell carcinoma of the tonsil. Head Neck 2012;34:613–616, PMID: 22250010.
9. O'Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys 2001;51:332–343, PMID: 11567806.
10. Jackson SM, Hay JH, Flores AD, et al. Cancer of the tonsil: the results of ipsilateral radiation treatment. Radiother Oncol 1999;51:123–128, PMID: 10435802.
11. Koo TR, Wu HG, Hah JH, et al. Definitive radiotherapy versus postoperative radiotherapy for tonsil cancer. Cancer Res Treat 2012;44:227–234, PMID: 23341786.
12. Rusthoven KE, Raben D, Schneider C, Witt R, Sammons S, Raben A. Freedom from local and regional failure of contralateral neck with ipsilateral neck radiotherapy for node-positive tonsil cancer: results of a prospective management approach. Int J Radiat Oncol Biol Phys 2009;74:1365–1370, PMID: 19168295.
13. Vergeer MR, Doornaert PA, Jonkman A, et al. Ipsilateral irradiation for oral and oropharyngeal carcinoma treated with primary surgery and postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:682–688, PMID: 20188492.
14. Chronowski GM, Garden AS, Morrison WH, et al. Unilateral radiotherapy for the treatment of tonsil cancer. Int J Radiat Oncol Biol Phys 2012;83:204–209, PMID: 22019242.
15. Cerezo L, Martin M, Lopez M, Marin A, Gomez A. Ipsilateral irradiation for well lateralized carcinomas of the oral cavity and oropharynx: results on tumor control and xerostomia. Radiat Oncol 2009;4:33PMID: 19723329.
16. Xia P, Fu KK, Wong GW, Akazawa C, Verhey LJ. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000;48:329–337, PMID: 10974445.
17. Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol 2006;24:2618–2623, PMID: 16763274.
18. Wu Q, Mohan R, Morris M, Lauve A, Schmidt-Ullrich R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys 2003;56:573–585, PMID: 12738335.
19. Toledano I, Graff P, Serre A, et al. Intensity-modulated radiotherapy in head and neck cancer: results of theprospective study GORTEC 2004-03. Radiother Oncol 2012;103:57–62, PMID: 22296746.
20. Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–136, PMID: 21236730.
21. Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;50:695–704, PMID: 11395238.
22. Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol 2006;78:270–275, PMID: 16564589.
23. Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent 2005;33:223–233, PMID: 15725522.
24. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004;60:1425–1439, PMID: 15590174.
Fig. 1(A, B) Patients with pT2N2b disease in the right tonsil. This patient underwent induction chemotherapy followed by tonsillectomy and modified neck dissection. Concurrent chemoradiotherapy was done as an adjuvant. Three months after the completion of radiotherapy, local failure occurred in the right tumor bed. 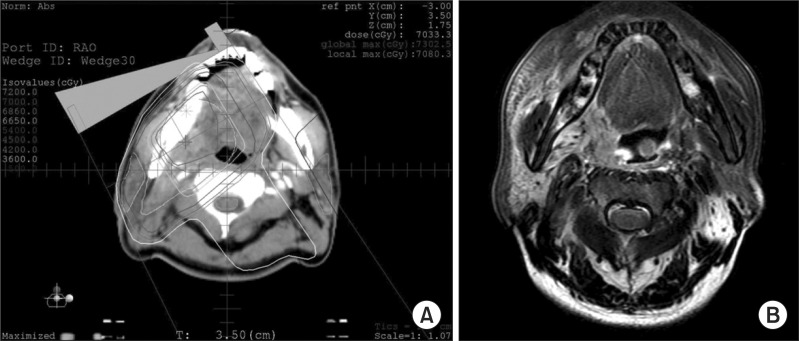
|
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |