Prediction of response by FDG PET early during concurrent chemoradiotherapy for locally advanced non-small cell lung cancer
Article information
Abstract
Purpose
To evaluate the predictive value of the early response of 18F-flurodeoxyglucose positron emission tomography (FDG PET) during concurrent chemoradiotherapy (CCRT) for locally advanced non-small cell lung cancer (NSCLC).
Materials and Methods
FDG PET was performed before and during CCRT for 13 NSCLC patients. Maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were measured and the changes were calculated. These early metabolic changes were compared with the standard tumor response by computed tomograms (CT) one month after CCRT.
Results
One month after the completion of CCRT, 9 patients had partial response (PR) of tumor and 4 patients had stable disease. The percent changes of SUVmax (%ΔSUVmax) were larger in responder group than in non-responder group (55.7% ± 15.6% vs. 23.1% ± 19.0%, p = 0.01). The percent changes of SUVmean (%ΔSUVmean) were also larger in responder group than in non-responder group (54.4% ± 15.9% vs. 22.3% ± 23.0%, p = 0.01). The percent changes of MTV (%ΔMTV) or TLG (%ΔTLG) had no correlation with the tumor response after treatment. All the 7 patients (100%) with %ΔSUVmax ≥ 50% had PR, but only 2 out of 6 patients (33%) with %ΔSUVmax < 50% had PR after CCRT (p = 0.009). Likewise, all the 6 patients (100%) with %ΔSUVmean ≥ 50% had PR, but only 3 out of 7 patients (43%) with %ΔSUVmean < 50% had PR after CCRT (p = 0.026).
Conclusion
The degree of metabolic changes measured by PET-CT during CCRT was predictive for NSCLC tumor response after CCRT.
Introduction
The most widely used molecular imaging for non-small cell lung cancer (NSCLC) is 18F-flurodeoxyglucose positron emission tomography (FDG PET) scan. 18F-FDG PET, using the differential glucose uptake and glycolysis between the cancer cells and normal cells, can provide valuable functional information of tumor. The PET-CT scan is a more advanced imaging modality than PET or CT alone because it combined functional and anatomical imaging.
The 18F-FDG PET-CT scan has become increasingly important in the staging and radiation therapy (RT) planning of NSCLC patients. The PET-CT is more sensitive and specific than other imaging modalities for detection of lymph node and distant metastasis [1]. The PET-CT could stage NSCLC more correctly than CT or PET alone [2]. 18F-FDG PET-CT scan can be used in planning of RT for delineating primary tumor volume and metastatic lymph node area. Several studies have shown that selective irradiation on involved lymph nodes can be done safely and effectively with PET-CT scan, with the isolated nodal failure rate of less than 5% [3,4].
About one-third of newly diagnosed NSCLC patients have locally advanced disease. Concurrent chemoradiotherapy (CCRT) is the standard of care for locally advanced NSCLC patients with good performance status. But one-third of these patients still experience local failure as their first site of relapse [5]. If the outcome of CCRT could be predicted during treatment, ineffective treatment with toxicities would be avoided and alternate therapy could be considered.
The predictive value of an early FDG PET response during chemotherapy has been established [6]. But less is known for the predictive value of an early PET response during radical CCRT or RT. Recently, it was reported by several investigators that the PET images taken as early as at the second week of treatment could predict the outcome of the treatment [7,8,9]. Therefore, we investigated retrospectively if the early metabolic response measured by 18F-FDG PET-CT scan during CCRT for NSCLC patients could predict tumor response one month after CCRT.
Materials and Methods
1. Patients and treatment methods
Thirteen patients with locally advanced NSCLC were included in this retrospective study. They were treated with CCRT in the Department of Radiation Oncology of the Boramae Medical Center between April 2012 and January 2014 and took the 18F-FDG PET-CT scan early during the CCRT. The PET-CT was taken only for patient who agreed to take the exam for the purpose of evaluating the tumor response during CCRT. The patient characteristics are listed in Table 1. The median follow-up period of 13 patients were 11 months (range, 6 to 30 months). Two patients expired at 11 months and 17 months of follow-up because of the progression of disease.
Chemotherapy consisted of 6 cycles of docetaxel (20 mg/m2) and cisplatin (20 mg/m2) given on days 1, 8, 15, 22, 29, 36, intravenously. The first cycle of chemotherapy was applied on the day 1 of RT. RT was given once daily with daily fraction 1.8 to 2.0 Gy, five days per week. Total dose of RT ranged from 59.4 Gy to 72 Gy (median dose, 64.5 Gy). RT was performed with 3-dimensional conformal therapy technique. One patient received 45 Gy of CCRT and underwent surgery (lobectomy and mediastinal lymph node dissection).
2. PET-CT
PET-CT scanning was performed using a dedicated PET-CT system (Gemini TF; Philips Healthcare, Cleveland, OH, USA). Patients were requested to fast at least 6 hours before PET-CT scanning, and FDG (5.18 MBq/kg) was intravenously injected after confirmation of blood glucose level (<140 mg/kg). At 1 hour after FDG injection, CT images (80 mA and 140 kVp) were obtained at a 5-mm section thickness from the skull base to the mid-thigh. CT images were reconstructed using a 512 × 512 matrix and a 50-cm field of view. And then, PET images were acquired from the mid-thigh to the skull base. PET images were reconstructed with a 128 × 128 matrix, using the ordered subset expectation maximum iterative reconstruction algorithm, an 8-mm Gaussian filter, and a 50-cm field of view. Acquired PET-CT images were transferred to a dedicated workstation and analyzed using a vendor-provided software (The Extended Brilliance Workspace with Fusion Viewer, Philips Healthcare).
A volume of interest (VOI) was placed on the primary tumor mass, and then PET-CT parameters were generated using a vendor-provided automated contouring program [10,11]. The automated contouring program set for a threshold with 60% of maximum standardized uptake values SUV (SUVmax) of the mass (Fig. 1). The PET-CT parameters of mean SUV (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were determined in the VOIs. The SUVs were calculated as follows:
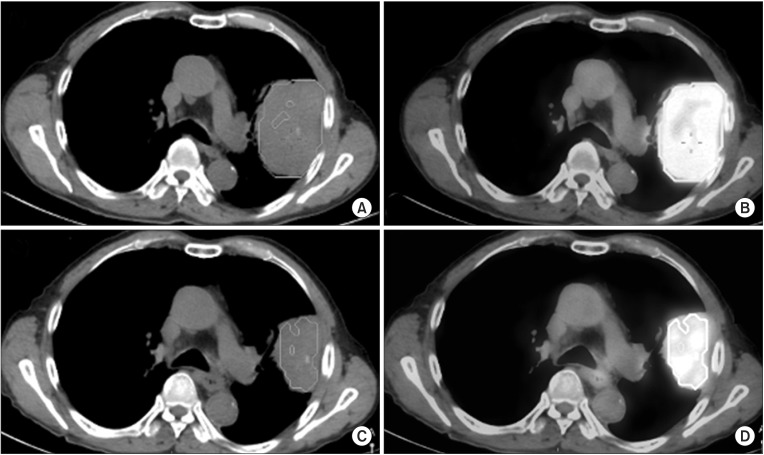
Measurement of the positron emission tomography-computed tomography (PET-CT) image parameters. A volume of interest with an isocontour at 60% of maximum standardized uptake value (SUVmax) was automatically drawn on the primary cancer mass in pre-therapy PET-CT (A and B), and post-therapy PET-CT (C and D), respectively.
SUV =
FDG activity concentration (Bq/mL) × total lean body weight (kg) / administered FDG activity (Bq)
MTV was defined as voxels presenting SUVs greater than thresholds within the auto-contouring margin. TLG was the product of SUVmean and MTV within the VOI.
The SUVmax, SUVmean, and TLG before CCRT and those during CCRT were obtained and the changes of those PET parameters were used for analysis.
ΔSUVmax and % ΔSUVmax were defined as following;
ΔSUVmax = (SUVmax before CCRT) - (SUVmax during CCRT)
%ΔSUVmax = ΔSUVmax × 100 / (SUVmax before CCRT)
ΔSUVmean, %ΔSUVmean, ΔMTV, %ΔMTV, ΔTLG, and %ΔTLG were calculated as with SUVmax.
We measure the SUV, MTV, and TLG of the primary tumor only and not measured those of the mediastinal lymph nodes, because the metabolic change of the primary tumor could represent the response of the lung cancer. According to the neoadjuvant treatment studies, the decrease of SUV of the primary tumor was more likely to predict the response of the lung cancer to CCRT than that of the mediastinal lymph node [12,13].
The tumor size before CCRT was the sum of the longest diameter of the primary tumor and the short axis of pathologic lymph nodes that had short axis ≥15 mm measured on the chest CT. The change of tumor size during CCRT was measured using the CT images of the PET-CT. ΔTumor size and %ΔTumor size were calculated as with PET-CT parameters.
The change of PET-CT parameters and the tumor response after CCRT were analyzed. The tumor response was evaluated according to the revised RECIST criteria (v.1.1) using the chest CT at 1 month after CCRT. For the one patient who received preoperative CCRT, the response was evaluated with both preoperative CT images and surgical specimens.
3. Statistics
To compare the PET-CT parameters before CCRT and those during CCRT, paired t-test was used. Student t-test was used to compare the changes of the SUVmax, SUVmean, MTV, TLG, and tumor size of the responder group and those of the non-responder group. χ2 test was performed to compare the response rate between two groups with different degree of changes in PET-CT parameters. All statistical analyses were performed using the SPSS ver. 20 software (IBM Corporation, Armonk, NY, USA).
Results
The SUVmax, MTV, SUVmean, TLG, and tumor size before and during CCRT are summarized in Table 2. The median SUVmax before CCRT was 14.1 (range, 9.6 to 17.3) and that during CCRT was 7.1 (range, 3.2 to 10.3). The median value of SUVmean before treatment were 7.9 (range, 5.6 to 12.6) and that during treatment were 5.0 (range, 2.3 to 7.4). The median tumor size before and during CCRT were 6.8 cm (range, 3.7 to 20.2 cm) and 4.8 cm (range, 2.4 to 10.1 cm), respectively. The SUVmax, MTV, SUVmean, TLG, and tumor size during CCRT were lower than those before CCRT and the differences were statistically significant.
One month after the completion of CCRT, 9 patients had partial response of tumor and 4 patients had stable disease. The response of the patient who received preoperative CCRT was partial response in both preoperative CT images and surgical pathology specimens. We compared the PET-CT parameters of the patients who had partial response after CCRT (responder group) and those of the patients who had only stable disease after treatment (non-responder group). The ΔSUVmax of all patients ranged from 0.4 to 12.6 with median value of 6.3. The %ΔSUVmax of all patients ranged from 4.3 to 77.5 with median value of 54.6. The ΔSUVmax of the responder group was 7.78 ± 0.73 and that of the non-responder group was 2.83 ± 0.53. There was statistically significant difference between the two groups (p = 0.01). The %ΔSUVmax of the responder group and that of the non-responder group showed significant difference, too (55.7 ± 15.6 vs. 23.1 ± 18.9, p = 0.01). The ΔSUVmean of all patients ranged from -0.3 to 9.4 with median value of 3.7. The %ΔSUVmean ranged from -4.9 to 74.6 with median value of 49.3. Table 3 shows that the ΔSUVmax, %ΔSUVmax, ΔSUVmean, and %ΔSUVmean of the responder group and those of the non-responder group were different with statistical significance. However, the ΔMTV, %ΔMTV, ΔTLG, and %ΔTLG of the responder group and those of the nonresponder group were not different significantly. ΔTumor size of the responder and non-responder group were not significantly different, but %ΔTumor size of the responder group and non-responder group were different with statistical significance (28.2 ± 18.3 vs. 11.8 ± 2.8, p = 0.03).

Comparison of the changes of SUVmax, SUVmean, MTV, TLG and tumor size of the responder group and those of the non-responder group
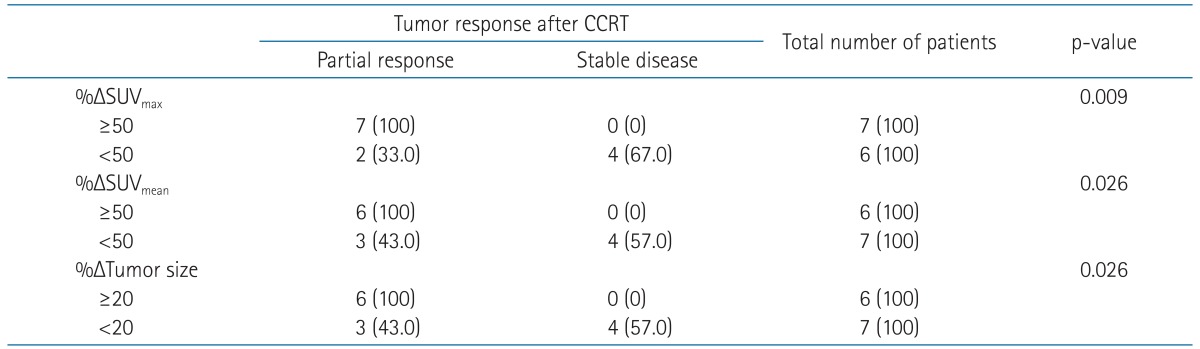
Table 4 shows the correlation between the early changes of SUVmax, SUVmean, tumor size and the tumor response after CCRT. Because the median values of the %ΔSUVmax and %ΔSUVmean were 54.6 and 49.3, respectively, we used %ΔSUVmax and %ΔSUVmean of 50 as cutoff value. If the %ΔSUVmax were 50 or larger, the tumors were more likely to achieve partial response after CCRT than the tumors with %ΔSUVmax less than 50 (p = 0.009). Likewise, the tumors with %ΔSUVmean ≥ 50 were more likely to achieve partial response after CCRT than the tumors with %ΔSUVmean less than 50 (p = 0.026). The correlation of the change of tumor size during CCRT and the tumor response after CCRT was analyzed, too. Because the median value of %ΔTumor size was 14, cutoff value of 20 was used. If the tumor size were decreased 20% or more during CCRT, the tumors were more likely to achieve partial response after treatment than those with less than 20% of decrease.
Discussion and Conclusion
The response to CCRT is known to be correlated with overall survival or progression-free survival in NSCLC patients [14]. Patients with complete or partial response had an improved overall survival rate compared with patients with stable or progressive disease. Progression-free survival was also improved significantly with response.
In this study, we showed that the early PET-CT response during CCRT could be helpful in predicting the response after CCRT. The change of tumor size during CCRT was also helpful in predicting the response after CCRT, but it's difficult to detect the 10%-30% change of tumor diameter early in the course of CCRT. It's more clear and obvious to detect the 50% change of SUV on PET images than to notice the minimal change of tumor size on CT images. The metabolic change of tumor could be detected sooner than the change of tumor size. If we could predict the tumor response early during CCRT, we might be able to take measures earlier to change the treatment strategy for the patient. It is possible to consider short course of CCRT and consolidative chemotherapy or surgical resection of tumor for patients with little or no response in early PET-CT examination.
Kong et al. [15] showed that there was significant correlation between the PET-CT response after 45 Gy of RT and the response 3 months after RT in 15 NSCLC patients who received RT alone or chemoradiotherapy. They measured normalized SUV (NSUV), which was defined as peak SUV of tumor divided by the mean SUV of the aortic arch. The NSUV reduction of primary tumors obtained from the during RT scans correlated significantly with that of the post-RT PET scans. In our study, the reduction of SUVmax or SUVmean after median dose of 21.6 Gy was correlated with the CT response 1 month after CCRT. Optimal timing for PET-CT examination during CCRT or RT is not well established yet, but earlier prediction of response during CCRT could be more useful and helpful for making proper decision. In addition, earlier PET-CT scan at 2nd to 4th week of CCRT can possibly avoid non-tumor-related effects, such as proliferation, cell repair, and inflammation [8].
Several studies have shown that there is a correlation between the SUV and tumor cell proliferation [16,17]. Therefore, the early reduction of FDG uptake during treatment can predict tumor response. In our study, the early changes of SUV during CCRT were predictive for tumor response after CCRT, but TLG was not. TLG is calculated as the product of SUV and MTV. According to a small study with 23 locally advanced NSCLC patients, early change of TLG during CCRT was correlated with progression-free survival [8].
In the present study, the correlation of PET-CT response during CCRT and overall survival could not be analyzed due to short follow-up period and small number of patients. But several studies have shown the prognostic significance of early PET-CT response in NSCLC patients.
van Elmpt et al. [9] found that early response assessment using 18F-FDG PET during radiotherapy was useful in predicting long-term survival of the advanced-stage NSCLC patients. They obtained PET scan images before RT and during the second week of RT. The average change in SUVmean and SUVmax inside the tumor volume was significantly different between the patients who survived 2 years and those who did not. When they used EORTC criterion of 15% for partial metabolic response, 2-year overall survival was 92% for patients with a decrease in SUVmean of more than 15% and 33% for those with a decrease in SUVmean of less than 15%.
Other investigators compared the %ΔSUVmax ≥ 50% group and %ΔSUVmax < 50% group as our study [18]. They obtained PET-CT images before treatment and during RT at the dose of 40-50 Gy for stage III NSCLC patients. The 2-year survival rate was 40% in the %ΔSUVmax ≥ 50% group and 37% in the %ΔSUVmax < 50% group. There was significant difference between the two groups (p = 0.001).
In conclusion, the early PET-CT examination was useful in predicting tumor response to CCRT for locally advanced NSCLC. But our study is limited by the small number of patients, short follow-up and retrospective study design. To confirm the correlation between early metabolic response and progressionfree survival or overall survival, prospective study with larger number of patients and longer follow-up period is needed in the future.
Notes
No potential conflict of interest relevant to this article was reported.