Patterns of failure and prognostic factors in resected extrahepatic bile duct cancer: implication for adjuvant radiotherapy
Article information
Abstract
Purpose
To find the applicability of adjuvant radiotherapy for extrahepatic bile duct cancer (EBDC), we analyzed the pattern of failure and evaluate prognostic factors of locoregional failure after curative resection without adjuvant treatment.
Materials and Methods
In 97 patients with resected EBDC, the location of tumor was classified as proximal (n = 26) and distal (n = 71), using the junction of the cystic duct and common hepatic duct as the dividing point. Locoregional failure sites were categorized as follows: the hepatoduodenal ligament and tumor bed, the celiac artery and superior mesenteric artery, and other sites.
Results
The median follow-up time was 29 months for surviving patients. Three-year locoregional progression-free survival, progression-free survival, and overall survival rates were 50%, 42%, and 52%, respectively. Regarding initial failures, 79% and 81% were locoregional failures in proximal and distal EBDC patients, respectively. The most common site was the hepatoduodenal ligament and tumor bed. In the multivariate analysis, perineural invasion was associated with poor locoregional progression-free survival (p = 0.023) and progression-free survival (p = 0.012); and elevated postoperative CA19-9 (≥37 U/mL) did with poor locoregional progression-free survival (p = 0.002), progression-free survival (p < 0.001) and overall survival (p < 0.001).
Conclusion
Both proximal and distal EBDC showed remarkable proportion of locoregional failure. Perineural invasion and elevated postoperative CA19-9 were risk factors of locoregional failure. In these patients with high risk of locoregional failure, adjuvant radiotherapy could be considered to improve locoregional control.
Introduction
Extrahepatic bile duct cancer (EBDC) is a rare cancer, which has less than 3% of proportion in all gastrointestinal malignancies [1]. EBDC can be divided into proximal and distal tumors by its location. Regardless of location of tumor, surgical resection is the treatment of choice [2,3]: combined hepatic and hilar resection for proximal tumor, and pancreaticoduodenectomy for distal tumor. However, complete resection with pathologically negative margin is difficult, because of its deep location and adjacent critical organs, such as major vessels.
The treatment outcomes of EBDC is generally poor, 5-year overall survival rates were 20% to 50%. Locoregional failure has been reported to be the most common type of initial failure in patients with EBDC. Then, it is possible to hypothesize that the addition of adjuvant radiotherapy can reduce locoregional failure and may have survival benefit. However, the evidence of adjuvant radiotherapy for EBDC has not been established yet, because there has been no randomized controlled study due to the low incidence of EBDC. Only small retrospective studies were published to evaluate the efficacy of adjuvant radiotherapy, but most studies did not encompass all subgroups of biliary tract, or included other sites, such as gallbladder or pancreas tumors.
In the present study, we tried to analyze the pattern of failure and evaluate prognostic factors in EBDC patients who underwent curatively resection without adjuvant treatment. Failure pattern analysis after curative intent surgery would support the rationale of adjuvant radiotherapy, and prognostic factor evaluation could contribute to find out which subgroup is expected to have better treatment outcomes with adjuvant radiotherapy.
Materials and Methods
Between May 2003 and December 2010, a total of 137 patients were pathologically diagnosed as adenocarcinoma in the extrahepatic bile duct or common bile duct at Seoul National University Bundang Hospital by surgery in curative intent. Of these patients, 31 were excluded as following reasons: history of prior malignancy (n = 15), postoperative mortality during in-hospital period (n = 7), no follow-up study (n = 5), double primary malignancy (n = 3), or chemotherapy before surgery (n = 1). Additionally, patients who underwent adjuvant treatment were excluded (n = 9), because adjuvant treatment was not routinely performed in our institution. Remaining 97 patients were included in the present study. All patients were restaged using the seventh edition of the American Joint Commission on Cancer TNM staging system [4]. We classified all EBDC into 2 subgroups by main tumor location, proximal and distal EBDC. Proximal EBDC was defined as the tumors located between the confluence of bilateral hepatic ducts and the junction of the cystic duct-common hepatic duct. In distal EBDC, tumors were located between the junction of the cystic duct-common hepatic duct and the ampulla of Vater.
The resectability of tumors was determined based on preoperative imaging studies, such as abdominal computed tomography (CT), magnetic resonance imaging, cholangiography, and choledochoscopy. Three types of surgery were conducted: extended hemihepatectomy and bile duct resection (for proximal tumors), segmental bile duct resection (for proximal tumors, limited in the common hepatic duct or bifurcation of right and left hepatic ducts), and pancreaticoduodenectomy (for distal tumors). Lymph nodes in the hepatodudenal ligament surrounding portal vein and hepatic artery were skeletonized, and lymph nodes on the right side of the celiac artery (CA) and the superior mesenteric artery (SMA) were removed as regional lymph nodes. The adequacy of resection margin was classified as complete resection without gross or microscopic tumor residue (R0), resection with microscopic tumor residue (R1), or gross tumor residue after resection (R2). All types of resection margins were included, if the surgery was performed with curative aim.
Medical records of all patients were reviewed and imaging studies from preoperative abdominal CT to postoperative follow-up abdominal CT were compared to determine the location of failure. The first follow-up abdominal CT was taken 1 to 3 months after surgery and then repeated every 3 months for 2 years followed by every 6 months afterwards. The location of locoregional failure was grouped as follows: the hepatoduodenal ligament and tumor bed, area around CA and SMA, and other sites (e.g., surgical wound). All sites of initial distant metastasis were also investigated. Tumor markers, carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen, were measured before and after surgery. Routinely, postoperative tumor markers were checked on one week, one month, and every three months for 2 years and then every six months after the surgery. Recurrent disease can result in the elevation of tumor marker level, as well as the postoperative microscopic or macroscopic residual disease. For the analysis of prognostic factors, the level of postoperative tumor markers in one month after the surgery was used to exclude the influence of recurrence.
Treatment-related toxicity was categorized according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4. Complication occurred within 3 months after surgery was defined as acute complication, and more than 3 months after surgery was as chronic complication.
For the statistical analysis between subgroups, the Pearson chi-square and the Fisher exact test were used. The Kaplan-Meier method was used to estimate survival rate. The log-rank test and the Cox proportional hazards regression model were used for the univariate and multivariate analysis, respectively. Factors with a p-value of less than 0.05 were regarded as statistically significant.
Results
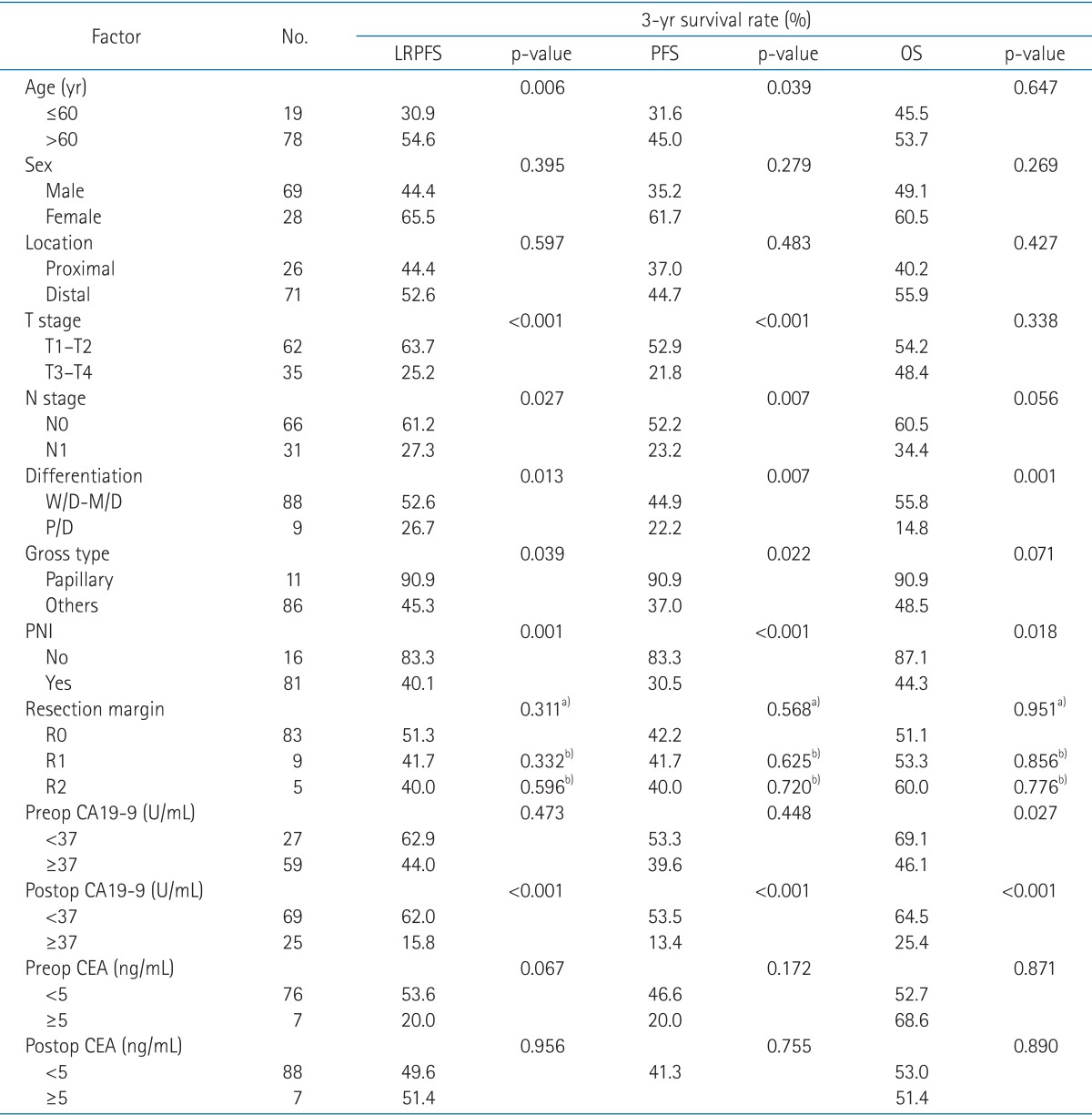
The median age was 69 years (range, 44 to 84 years). Twenty-six patients (26.8%) had proximal EBDC and 71 patients (73.2%) had distal EBDC. Regarding surgery, the pancreatoduodenectomy was the most common type (distal tumor, n = 53), followed by the segmental bile duct resection (proximal tumor, n = 6; distal tumor, n = 18) and the extended hemihepatectomy (proximal tumor, n = 20). Regarding tumor gross type, 11 patients (11.3%) had papillary type, 58 patients (59.8%) had nodular type, 24 patients had flat type (24.7%), and 4 patients (4.1%) had infiltrating-ulcerative type. Tumor differentiation was as follows: 14 cases (14.4%) were well differentiated, 74 cases (76.3%) were moderately differentiated, and 9 cases (9.3%) were poorly differentiated. Characteristics of patients and tumors were listed in Table 1.
The median follow-up time was 29.0 months (range, 5.2 to 78.0 months) for the surviving patients. The median locoregional progression-free survival (LRPFS) was 24.7 months for all patients, 24.7 months for proximal EBDC patients, and 37.3 months for distal EBDC patients. The median overall survival (OS) and progression-free survival (PFS) times were 37.0 and 22.5 months for all patients, 25.7 and 12.6 months for proximal EBDC patients, and 45.6 and 23.5 months for distal EBDC patients, respectively. There was no significant difference between tumor locations in OS (p = 0.427), PFS (p = 0.483), and LRPFS (p = 0.597).
In the univariate analysis (Table 1), following factors had statistically significant association with LRPFS: age (p = 0.006), T stage (p < 0.001), N stage (p = 0.027), tumor differentiation (p = 0.013), gross type (p = 0.039), perineural invasion (PNI; p = 0.001), and postoperative CA19-9 (p < 0.001). For PFS, there was a significant association in age (p = 0.039), T stage (p < 0.001), N stage (p = 0.007), tumor differentiation (p = 0.007), gross type (p = 0.022), PNI (p < 0.001), and postoperative CA19-9 (p < 0.001). Tumor differentiation (p = 0.001), PNI (p = 0.018), preoperative CA19-9 (p = 0.027) and postoperative CA19-9 (p < 0.001) were associated with OS.
Multivariate analysis of tumor factors, including T stage, N stage, tumor differentiation, gross type, PNI, and postoperative CA19-9 was performed (Table 2). PNI and postoperative CA19-9 were independent prognostic factors of LRPFS (p = 0.023 and p = 0.002) and PFS (p = 0.012 and p < 0.001). Regarding OS, tumor differentiation (p = 0.011) and postoperative CA19-9 (p < 0.001) had significant associations. Well-known prognostic factors, such as T stage and N stage, did not have significant associations with locoregional failure and survival.
Modified multivariate analysis was performed excluding postoperative CA19-9, which had most strong statistical power. Prognostic factors of LRPFS were T stage (p = 0.002; relative ratio [RR], 2.640; 95% confidence interval [CI], 1.416-4.923), tumor differentiation (p = 0.019; RR, 2.950; 95% CI, 1.193-7.294), and PNI (p = 0.030; RR, 9.132; 95% CI, 1.236-67.497). Similarly, prognostic factors of PFS were T stage (p = 0.003; RR, 2.496; 95% CI, 1.379-4.517), tumor differentiation (p = 0.016; RR, 2.787; 95% CI, 1.211-6.416), and PNI (p = 0.017; RR, 11.356; 95% CI, 1.540-83.719). Regarding OS, tumor differentiation (p = 0.008; RR, 3.104; 95% CI, 1.347-7.154) and PNI (p = 0.045; RR, 3.376; 95% CI, 1.029-11.069) showed significant association.
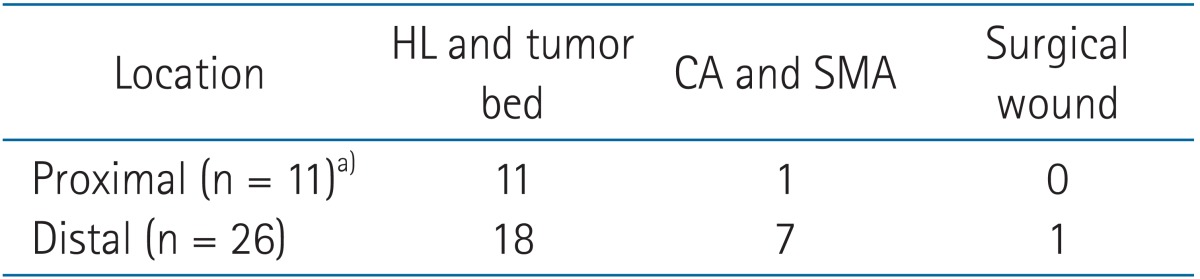
Among initial failures, 79% and 81% were locoregional failures in proximal and distal EBDC patients, respectively. Significant difference in initial patterns of failure by the tumor locations of EBDC was not found (p = 0.702) (Table 3). The hepatoduodenal ligament and tumor bed was the most common site of locoregional failure, in all locations of EBDC (Table 4). Distant metastasis occurred in the liver most commonly in both groups of EBDC (Table 5). Regarding the relationship between the resection margin and locoregional failure, there was no difference. Locoregional failures occurred in 41.0%, 55.6%, and 60.0% of the patients with R0, R1, and R2, respectively (p = 0.521).
Acute adverse effects of grade 3 or more were noted in 27 patients (27.8%). The most common was surgical wound problem, followed by cholangitis, biliary sepsis, liver abscess, complicated fluid collection, and anastomosis leakage/bleeding. One patient expired from septic shock due to liver abscess and complicated fluid collection. Three months or more after operation, 15 patients (15.5%) experienced grade 3 or more chronic adverse effects. Operation-related infections (cholangitis, biliary sepsis, and liver abscess) were majorities; others were incisional hernia, small bowel strangulation, and anastomosis bleeding. One patient underwent emergency operation due to complicated fluid collection and hernia, but expired.
Discussion and Conclusion
The evidence of adjuvant radiotherapy for EBDC has not been established because of its rareness. Several retrospective studies of adjuvant radiotherapy with or without chemotherapy were conducted to overcome the poor prognosis of EBDC. However these studies had potential risk of selection bias, for example, the patients with poor characteristics might receive adjuvant radiotherapy. No patient in the current study underwent adjuvant radiotherapy. This might be a limitation of this study, because it was impossible to compare the outcomes between adjuvant radiotherapy group and surgery alone group. On the contrary, prognostic factors of LRPFS in the current study could be more meaningful due to the analysis of no adjuvant radiotherapy treated cases. Another point of this study was that tumor location of EBDC was analyzed as one of prognostic factors.
In the present study, the 3-year LRPFS rates showed no difference (p = 0.597) between proximal and distal EBDCs. Locoregional failure was the most common pattern of initial failure, and tumor location did not have an influence on pattern of failure. Similar results were reported by Jarnagin et al. [5]. They analyzed 97 patients with gallbladder cancer (GBCA) and 80 patients with hilar cholangiocarcinoma (HCCA) who underwent potentially curative resection. A total of 18 patients (11 with GBCA and 7 with HCCA) received adjuvant therapy. Initial locoregional failure among all recurrences occurred in 21 patients (28%) with GBCA and 38 patients (65%) with HCCA. Considering initial locoregional failure of HCCA, the authors concluded that adjuvant radiotherapy could have rationale to delay the appearance of locoregional disease recurrence. Conversely, Park et al. [6] analyzed 101 patients with resected EBDC who underwent adjuvant radiotherapy. Isolated locoregional failure occurred in 7 patients (12%), and synchronous locoregional failure and distant metastasis in 11 patients (19%). Distant metastasis alone occurred in 40 patients (69%). Different from the report by Jarnagin et al. [5], locoregional failure denoted a minor proportion of recurrence. This difference would result from the point that adjuvant radiotherapy was given to all patients and may enhance locoregional control in their study. A comparison study, by Gwak et al. [7] noted that adjuvant radiotherapy decreased locoregional failure. The authors compared outcomes of surgery alone (group I) and surgery followed by adjuvant radiotherapy (group II) in the patients with EBDC. Local failure rate was decreased in the group II (62% vs. 36%; p = 0.02), while no difference was found in the 5-year OS rates (12% vs. 21%; p > 0.5).
Several studies [5,6,8] reported that locoregional failure was found in the hepatoduodenal ligament, the retroperitoneal lymph nodes, and the tumor bed. Generally, the field of adjuvant radiotherapy includes the bile duct and potential lymphatic drainage areas (lymph nodes around the hepatoduodenal ligament, CA, and SMA) as clinical target volume [9,10]. In the current study, most locoregional failure occurred in the hepatoduodenal ligament and tumor bed, regardless of the location of EBDC. However, locoregional failure in lymph nodes around the CA and SMA represented in 7 patients (27% of total locoregional failure) of distal EBDC, while in 1 patient (9% of total locoregional failure) of proximal EBDC. Based on failure pattern analysis of this study, it could be suggested that it is not mandatory to encompass the area around CA and SMA for radiotherapy portal in the patients with proximal EBDC. By modifying the radiotherapy volumes as above, bowel toxicity, the most common adverse effects of radiotherapy for gastrointestinal cancers could be avoided.
In this study, postoperative CA19-9 was a prognostic factor local control and survival in EBDC patients. CA19-9 is a carbohydrate tumor-associated antigen originally isolated from the culture medium of a human colorectal cancer cell line. For the patients with pancreatic cancer, postresection CA19-9 level is revealed as a highly significant predictor of OS in a prospective phase III trial [11]. However, the role of CA19-9 as a prognostic factor in EBDC has not been investigated clearly. A retrospective study reported that elevated postoperative CA19-9 level was an independent predictor of OS and PFS [6]. When measuring the level of CA19-9 after adjuvant radiotherapy, the patients whose CA19-9 level was normalized had better survival than the patients whose CA19-9 level remained elevated. Kim et al. [12] reported that postoperative CA19-9 showed significant association with locoregional control, disease-free survival, and OS in the univariate analysis, though not included in the multivariate analysis. The microscopic residual disease after curative surgery may be a reason of elevated postoperative CA19-9 level, and could behave as the origin of recurrence, as well.
PNI was another factor which had significant association with LRPFS and PFS, while showed a tendency of association with OS in multivariate analysis. The biliary tree has an extensive neural system, which consists of autonomic nerves mainly. Though the mechanism of tumor cells involving nerve fibers surrounding the biliary system has not been identified clearly, high rates of PNI has been reported in cholangiocarcinoma, approximately 75% to 85% [13,14,15]. In this study, PNI was noted in 84% of patients, and associated with prominent locoregional failure. The role of PNI as a prognostic factor in EBDC has not been proved well, but several studies reported that PNI indicated poor prognosis in the patients with cholangiocarcinoma [13,14,16]. These findings suggest that the patients with PNI will be needed to receive adjuvant treatment, to reduce the risk of locoregional failure. For similar example in head and neck cancer patients, the status of PNI is a required element in the pathologic report according to the Cancer Protocols and Checklists by the College of American Pathologists, because PNI is associated with poor locoregional control and survival [17]. The current study, though retrospective review, may contribute to establish the prognostic role of postoperative CA19-9 and PNI as an indication of poor locoregional control in EBDC patients.
Interestingly, well-known prognostic factors of EBDC, such as T stage, N stage, and tumor differentiation, did not show significant association with locoregional failure and survival in this study. It is speculated that the statistical power of postoperative CA19-9 overwhelmed other factors too much. In supporting this hypothesis, modified multivariate analysis with the exclusion of postoperative CA19-9 showed that higher T stage and poorly differentiated tumor were associated with poorer LRPFS (p = 0.002 and p = 0.019) and PFS (p = 0.003 and p = 0.016), respectively.
Another well-known prognostic factor, the status of resection margin was not a significant prognostic factor in the current study either. The patients with R0 and R1/2 did not have discrepancy in the pattern of locoregional failure. Furthermore, when comparing R1 and R2, no difference was found. At three years, between R1 and R2 groups, LRPFS rates were 42% and 40% (p = 0.770), and OS rates were 53% and 60% (p = 0.962), respectively. However, several studies has been reported that R1 had comparable prognosis with R0, while R2 had the worst prognosis, when adjuvant radiotherapy was performed [6,18,19]. A hypothesis that adjuvant radiotherapy might improve locoregional control with eradicating microscopic disease could be supported by these results. In this way, our results, no significant difference in prognosis among the resection margin statuses, might be explained by the absence of adjuvant treatment. Also, relatively short follow-up time and population disparity between R0 and R1/2 patients might be reasons.
In summary, we analyzed the patterns of failure and evaluated the prognostic factors in EBDC patients. Locoregional failure was the most common type of failure and the hepatoduodenal ligament and tumor bed was the most common site. Though the influence of adjuvant radiotherapy on survival was in conflict [7,8,12,19,20], it can be expected that the use of adjuvant radiotherapy could improve locoregional control considering remarkable rates of locoregional failure in EBDC. It is also needed to consider PNI and postoperative CA19-9 as prognostic factors besides the well-known pathological risk factors, such as resection margin, tumor staging, nodal involvement, and histologic differentiation.
Notes
No potential conflict of interest relevant to this article was reported.