Treatment outcomes of neoadjuvant concurrent chemoradiotherapy followed by esophagectomy for patients with esophageal cancer
Article information
Abstract
Purpose
To evaluate treatment outcomes and determine prognostic factors in patients with esophageal cancer treated with esophagectomy after neoadjuvant chemoradiotherapy (NCRT).
Materials and Methods
We retrospectively evaluated 39 patients with esophageal cancer who underwent concurrent chemoradiotherapy followed by esophagectomy between 2002 and 2012. Initial clinical stages of patients were stage IB in 1 patient (2.6%), stage II in 5 patients (12.9%), and stage III in 33 patients (84.6%).
Results
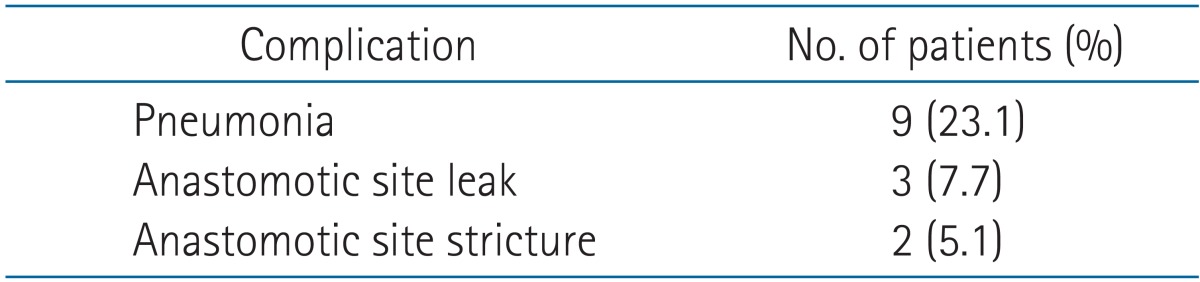
The median age of all the patients was 62 years, and the median follow-up period was 17 months. The 3-year overall survival (OS) rate was 33.6% in all the patients. The 3-year locoregional recurrence-free survival (LRFS) rate was 33.7%. In multivariate analysis with covariates of age, the Eastern Cooperative Oncology Group performance status, hypertension, diabetes mellitus, tumor length, clinical response, clinical stage, pathological response, pathological stage, lymphovascular invasion, surgical type, and radiotherapy to surgery interval, only pathological stage was an independent significant prognostic factor affecting both OS and LRFS. The complications in postoperative day 90 were pneumonia in 9 patients, anastomotic site leakage in 3 patients, and anastomotic site stricture in 2 patients. Postoperative 30-day mortality rate was 10.3% (4/39); the cause of death among these 4 patients was respiratory failure in 3 patients and myocardial infarction in one patient.
Conclusion
Only pathological stage was an independent prognostic factor for both OS and LRFS in patients with esophageal cancer treated with esophagectomy after NCRT. We could confirm the significant role of NCRT in downstaging the initial tumor bulk and thus resulting in better survival of patients who gained earlier pathological stage after NCRT.
Introduction
In Korea, esophageal cancer was the 16th most commonly diagnosed cancer in 2011 and the ninth most common cause of cancer-related deaths [1]. Esophageal cancer that is truly confined to the primary site is found in 20% of patients [2]. However, most patients are diagnosed with locally advanced esophageal cancer, and for them, definitive concurrent chemoradiotherapy (CCRT) has been the standard treatment. We previously reported the results of definitive chemoradiotherapy (DCRT) for locally advanced esophageal cancer [3,4,5,6]. However, a significant proportion of patients (40%-60%) still developed locoregional recurrence after DCRT [3,4,5,6,7,8,9,10], urging investigations into multimodality therapy that combines surgery with neoadjuvant chemoradiotherapy (NCRT). NCRT in esophageal cancer could enhance curative resection and eradicate micrometastases, thereby eventually improving overall survival (OS) and disease-free survival. Recently, two randomized trials have shown an increase in the OS associated with NCRT followed by surgical resection, compared to surgery alone [11,12]. A recent update of the CROSS trial showed that locoregional recurrence after surgery alone was significantly higher than that after NCRT plus surgery [13]. Furthermore, histology of squamous cell carcinoma significantly increased the risk of developing locoregional recurrence in the surgery alone arm, while there was no significant difference between squamous cell carcinoma and adenocarcinoma in the NCRT plus surgery arm [13]. Three meta-analyses have demonstrated that NCRT improved the pathological response rate, local and regional control, and the 3-year OS, compared with surgery alone [14,15,16]. Although two studies evaluating the role of trimodality therapy compared to DCRT alone showed no survival advantages [17,18], other studies examining salvage surgery after DCRT reported prolonged survival in carefully selected patients with local relapse [19,20,21]. Therefore, we performed a retrospective analysis to evaluate treatment outcomes and determine prognostic factors in patients with esophageal cancer treated with esophagectomy after NCRT.
Materials and Methods
1. Study patients
We reviewed 39 patients with esophageal cancer treated with NCRT followed by planned esophagectomy at Chonnam National University Hospital between 2002 and 2012. The pretreatment staging workup included a physical examination, esophagogastroscopy and biopsy, endoscopic ultrasonography (EUS), chest and abdominal computed tomography (CT), esophagography, and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), if necessary. All patients were restaged according to the guidelines of the American Joint Committee on Cancer (AJCC), 7th edition, for the TNM classification of esophageal cancer.
2. Treatment
1) Radiotherapy
External beam radiotherapy (RT) was performed using a 3-dimensional technique with LINAC 6- or 10-MV X-rays. The gross tumor volume (GTV) was defined as all detectable primary tumors and the involved lymph nodes. The clinical target volume included the mediastinum and esophagus within a 3- to 5-cm cephalocaudal margin and a 1.5- to 2-cm radial margin from the GTV. The elective nodal irradiation field was defined according to the primary tumor site. For tumors involving the proximal third of the esophagus or those proven to have supraclavicular lymph node adenopathy, the supraclavicular fossa was included. If the GTV had invaded the esophagogastric junction or the distal third of the esophagus, then the cardiac and celiac nodes were included. The prescribed RT dose was 44.0-50.4 Gy, administered in 1.8 or 2 Gy fractions.
2) Chemotherapy
Chemotherapy was administered concurrently on the first day of RT. Most patients underwent platinum-based CCRT every 4 weeks, with 5-fluorouracil (1,000 mg/m2) as a bolus infusion on day 1 to 4, and cisplatin (75 mg/m2) as an intravenous infusion for 4 hours on day 1. As part of a clinical trial in our institution, some patients received weekly cisplatin (25 mg/m2) combined with docetaxel (20 mg/m2), which was administered as a 3-hour intravenous infusion on days 1, 8, and 15.
3) Surgery and adjuvant treatment
Surgery was scheduled for 6 to 8 weeks after NCRT. Incomplete resection was determined by the presence of positive margins of microscopic examination (R1) or residual gross disease (R2). Patients who had incomplete surgical resection or who showed pathologically adverse findings, such as positive nodes, received postoperative adjuvant chemotherapy within 8 weeks of surgical resection.
3. Evaluation of CCRT response and treatment toxicity
The response to CCRT was evaluated with esophagography, esophagoscopy, bronchoscopy, CT, EUS, and PET, if necessary, before surgery. Response evaluation was performed according to the Response Evaluation Criteria in Solid Tumors guidelines [22]. The clinical response to CCRT was evaluated by comparing pre-CCRT CT images with those obtained post-CCRT or just before surgery. Pathologic response evaluation was performed by analyzing surgical specimens. A pathologic complete response (pCR) was defined the absence of histologically identifiable residual cancer and fibrosis extending through the different layers of the esophagus. Postoperative complication was defined as complication occurring 'in postoperative day 90'. Postoperative mortality was defined as death occurring 'in-hospital' or 'in postoperative day 30.'
4. Statistical analysis
Patients were followed-up periodically until the last follow-up or death. OS was defined as the time from the initiation of RT to the last follow-up or death from any cause. Locoregional recurrence-free survival (LRFS) was calculated from the start date of RT to the date of locoregional recurrence after surgery or death from any cause. Survival curves were generated using the Kaplan-Meier method and compared via the log-rank test. The Cox proportional hazards model was used for multivariate analysis of variables predicting survival. All statistical analyses were performed using SPSS ver. 21 (IBM SPSS Inc., Armonk, NY, USA), and p-values less than 0.05 were considered statistically significant.
Results
1. Patient and tumor characteristics
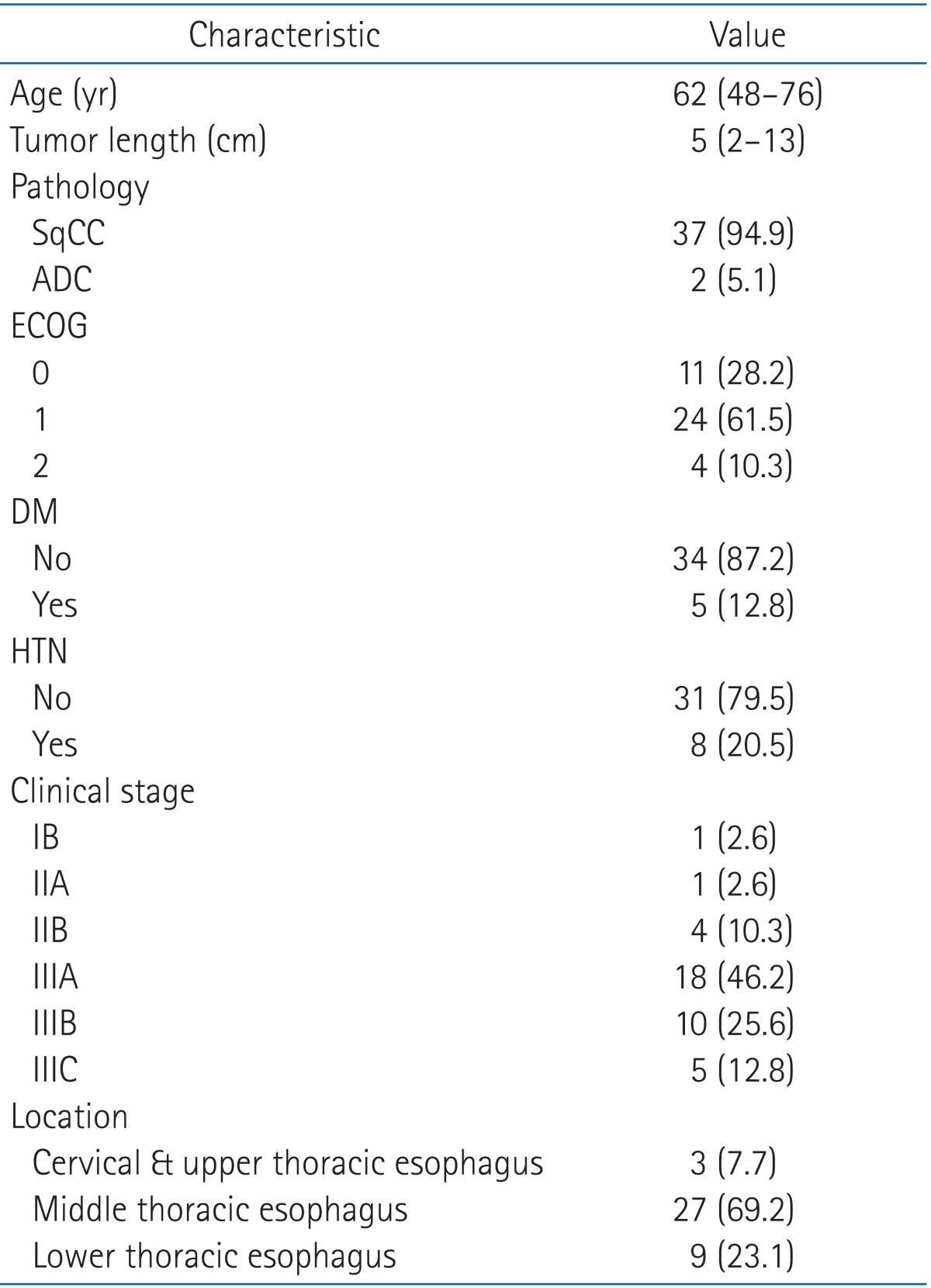
All patients were men, with a median age of 62.0 years (range, 48 to 76 years). Most patients had squamous cell carcinoma (n = 37, 94.9%). Based on the AJCC 7th edition staging system, the clinical stages of our patients before CCRT were stage IB in 1 patient (2.6%), stage II in 5 patients (12.9%), and stage III in 33 patients (84.6%). On examining the primary tumor location, cervicothoracic and upper thoracic tumors were found in 3 patients (7.7%), while middle and lower thoracic tumors were found in 27 patients (69.2%) and 9 patients (23.1%), respectively. Other detailed characteristics are described in Table 1.
2. Treatment characteristics
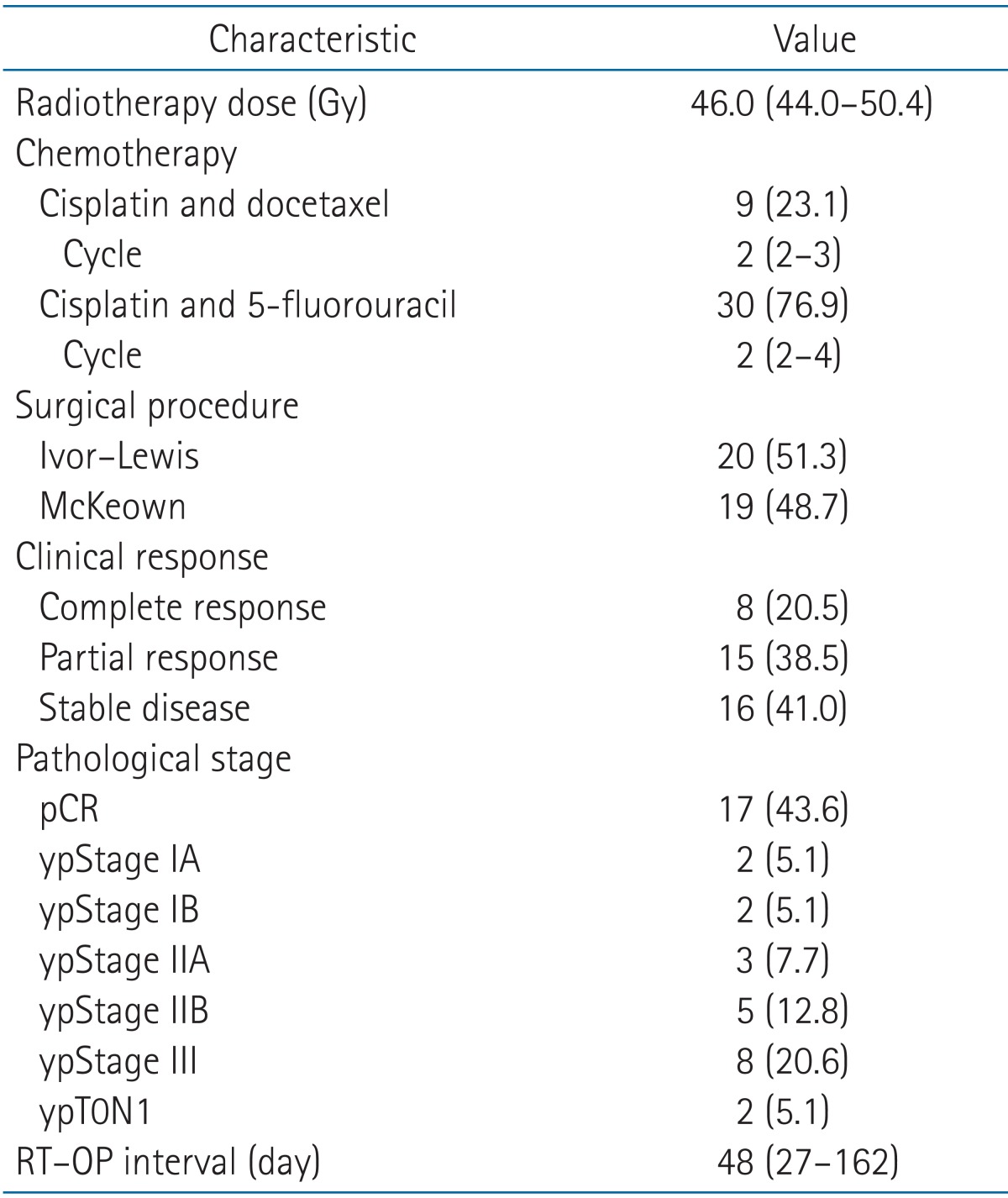
The actual delivered RT dose ranged from 44.0 to 50.4 Gy (median, 46.0 Gy). The concurrent chemotherapy regimen cisplatin and 5-fluorouracil was administered to 30 patients (76.9%) and cisplatin plus docetaxel was administered to 9 patients (23.1%). Surgery was performed 4 to 24 weeks (median, 7 weeks) after NCRT. Fourteen patients (35.9%) had more than an 8-week interval from completion of CCRT to surgery. The reasons for the long interval were unplanned additional chemotherapy (5 patients), acute toxicity of CCRT (2 patients), mesentery artery evaluation for colon interposition for surgery (2 patients), and unknown reasons (5 patients). Of the 39 patients, complete resection (R0 resection) was successfully accomplished in 38 patients (97.4%). One patient had microscopic residual disease (R1). Twenty patients (51.3%) underwent the Ivor-Lewis procedure with two field lymph node dissection, and 19 patients (48.6%) were treated using the McKeown approach. Fourteen patients in entire cohort showed pathologically positive lymph nodes after lymph node dissection. Of them, seven patients received postoperative adjuvant chemotherapy. One patient who had incomplete resection (R1) received adjuvant chemotherapy. All other treatment characteristics are shown in Table 2.
3. CCRT response and survivals
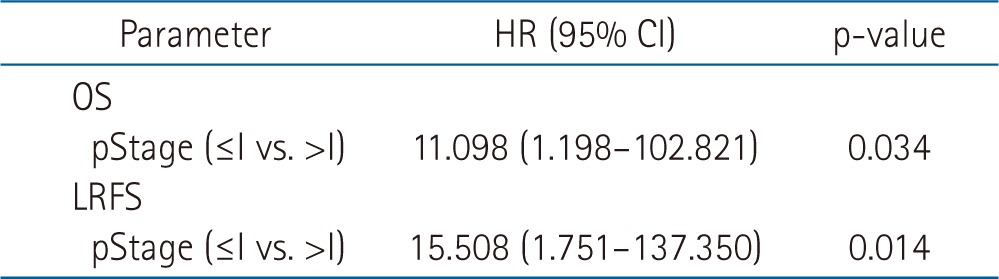
The median follow-up time was 17 months (range, 4 to 90 months). A pCR was observed in 17 patients (43.6%) of all patients. In entire 39 patients, a clinical complete response was observed in 8 patients, 3 of whom had a pCR. Furthermore, 31 patients had a clinical non-complete response, 14 of whom had a pCR. The pathologic stages after surgery were pCR in 17 patients (43.6%), ypStage I in 4 patients (10.2%), ypStage II in 8 patients (20.5%), ypStage III in 8 patients (20.6%), and ypT0N1 in 2 patients (5.1%). The 3-year locoregional control rate was 59.2% in all patients. The median OS was 19 months and the 3-year OS rate was 33.6% in all patients (Fig. 1). The median LRFS was 17 months and the 3-year LRFS rate was 33.7% in all patients (Fig. 1). By performing univariate analysis with covariates of age (≤65 years vs. >65 years), Eastern Cooperative Oncology Group (ECOG) performance status (0-1 vs. 2), hypertension, diabetes mellitus, tumor length (≤5 cm vs. >5 cm), clinical response, clinical stage, (≤II vs. >II), pathological response, pathological stage (≤I vs. >I), lymphovascular invasion, surgical type (Ivor-Lewis vs. McKeown), and radiotherapy to surgery interval (≤8 weeks vs. >8 weeks), there was no statistically significant factor for OS, but ECOG performance score was only a significant prognostic factor for LFRS in univariate analysis (Table 3). By multivariate analysis, only pathological stage was an independent significant prognostic factor affecting both OS and LRFS (Fig. 2, Table 4).
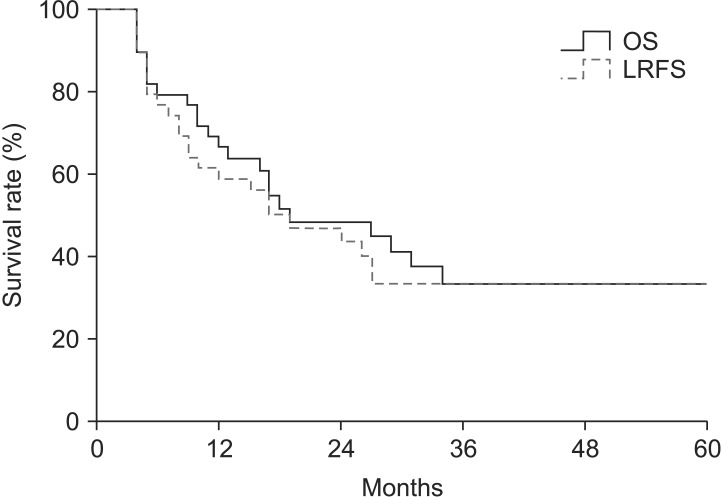
Overall survival (OS) and locoregional recurrence-free survival (LRFS) rate curves in entire patients, which show the 3-year OS and LRFS rates of 33.6% and 33.7%, respectively.
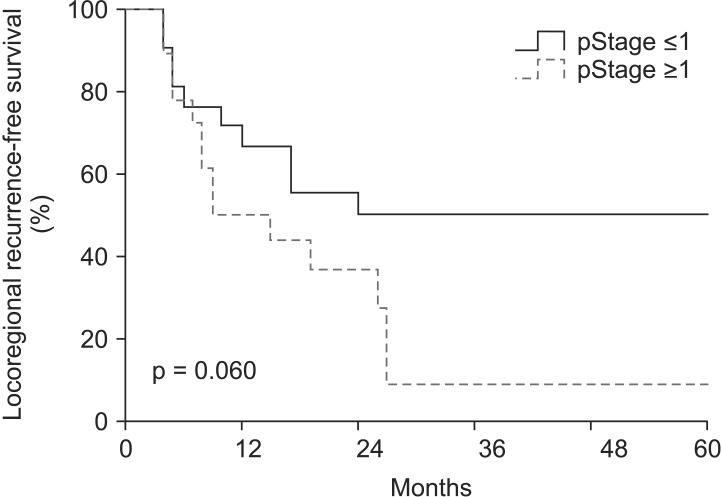
Locoregional recurrence-free survival (LRFS) rate in entire patients according to the pathological stage (pStage); the 3-year LRFS rates of pStage ≤I (n = 21) and >I (n = 18) were 50.0% and 9.1%, respectively (p = 0.06).
4. Patterns of failure and salvage treatment
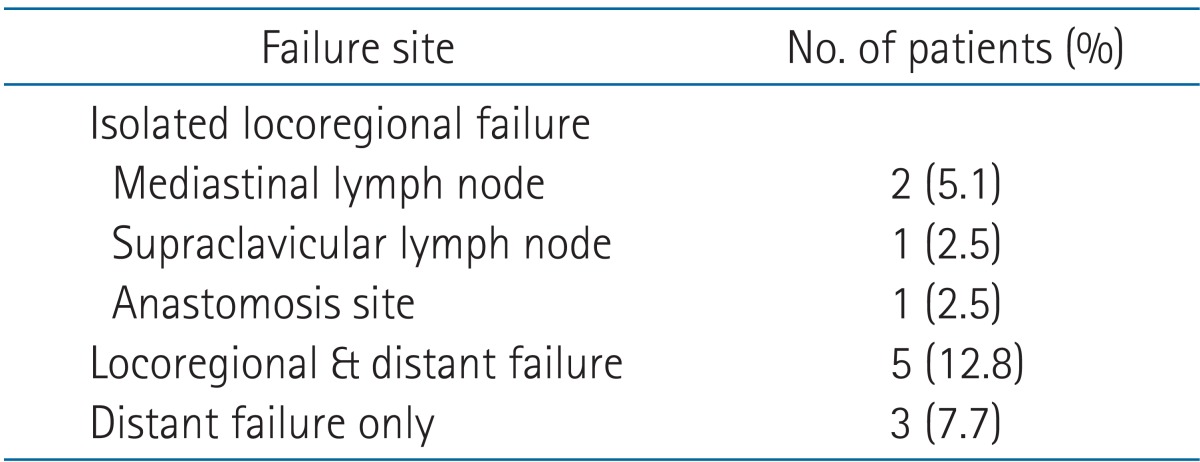
Isolated locoregional failure developed in 4 patients (10.1%). Three patients had regional nodal failure, and 1 patient had failure at the anastomosis site (Table 5). Distant sites of metastasis included the lung (n = 4), para-aortic nodes (n = 2), bone (n = 2), and adrenal gland (n = 1). Salvage CCRT was performed in 1 patient who had cervical lymph nodes metastases. Salvage chemotherapy was performed in 8 patients; 2 patients had isolated locoregional failure, 5 had locoregional and distant failure, and one had distant failure only. Three patients who could not undergo any type of salvage therapy received the best supportive care.
5. Treatment-related complications and mortality
The complications in postoperative day 90 were pneumonia (9 patients), anastomotic site leakage (3 patients), and anastomotic site stricture (2 patients) (Table 6). Postoperative 30-day mortality rate was 10.3% (n = 4); the cause of death among these 4 patients was respiratory failure (3 patients) and myocardial infarction (1 patient). Based on the RT to surgery interval, the postoperative mortality rate was no statistically significant differences who had more than an 8-week interval from completion of CCRT to surgery than in patients with a shorter interval (2 patients [14.3%] vs. 2 patients [8.0%], respectively; p = 0.535).
Discussion and Conclusion
One meta-analysis study recently updated the results from randomized trials regarding neoadjuvant chemotherapy or NCRT [15]. The authors reported that hazard ratio (HR) for all-cause mortality for NCRT plus surgery was significantly lower compared to surgery alone in patients with squamous cell carcinoma or with adenocarcinoma as well. The HR for all-cause mortality for neoadjuvant chemotherapy was low for patients with adenocarcinoma only (HR, 0.83; 95% CI, 0.71-0.95; p = 0.01), but not for patients with squamous cell carcinoma (HR, 0.92; 95% CI, 0.81-1.04; p = 0.18). A recent update of the CROSS trial showed that locoregional recurrence after surgery alone was significantly higher than that after NCRT plus surgery, more prominently in patients with histology of squamous cell carcinoma [13]. Earlier one study reviewed six randomized controlled trials (RCTs) comparing NCRT plus surgery with surgery alone [14]. Of six RCTs, four RCTs available for evaluating the downstaging effect of NCRT showed that patients who had received NCRT were less likely to have an advanced stage of cancer at pathological examination than were controls. The authors concluded that NCRT plus surgery significantly reduced 3-year mortality compared with surgery alone in either patients with squamous cell carcinoma or adenocarcinoma.
Our data indicate that having a pathologic stage ≤I was an independent prognostic factor for both OS and LRFS in all patients treated with NCRT plus surgery. Berger et al. [23] showed that having a pathologic stage ≤I correlated with an improved OS and disease-free survival compared with those patients who were not downstaged after NCRT. The ECOG performance status was also a significant variable affecting LRFS in our study albeit on univariate analysis. A better ECOG performance score correlated with a lower tumor burden and could allow the administration of multimodality treatments as well. Kim et al. [24] reported that among the patients who underwent esophagectomy, the group with a good performance status, clinical stage II tumor, and a major pathologic response to CCRT had the most favorable prognosis. Our recent study on DCRT also showed that the ECOG performance status was a significant factor affecting survival [3]. Our study showed that the 3-year OS and LRFS rates for patients treated with NCRT plus surgery were 33.6% and 33.7%, respectively, seemingly a little higher than our previously published results of 20.7%-33.0% with DCRT alone [3,4,5,6], although a direct comparison between the studies is not possible because they are retrospective analyses. Nevertheless, patients in this study showed mostly locally advanced stage III disease (84.6%), similar to those observed in previous DCRT studies [3,4,5,6]. However, other prospective studies comparing trimodality therapy to DCRT alone showed no survival advantages, albeit in a small number of patients [17,18].
Our study showed that the complete resection rate was 97.4% in all the patients, which was comparable to the 92% reported by the randomized controlled CROSS trial [12]. Schneider et al. [25] performed a study evaluating the response to trimodality therapy and found that the 3-year survival rate of R0 patients was 54%, while all non-R0 resection patients died within 3 years. In our study, one patient with R1 resection died at 13 months despite salvage chemotherapy. In one systematic review regarding the benefits and risks of NCRT for esophageal cancer, the mean pCR rate was reported to be 25.8% across all reviewed studies [26]. The pCR rate in this study was 43.6% in the entire patient cohort, which was comparable to that of squamous cell carcinoma patients in the CROSS trial [12]. However, the pCR rate of adenocarcinoma patients in the CROSS trial was 23%, while the 2 adenocarcinoma patients in our study did not achieve a pCR. Achieving a pCR has been known to be a powerful prognostic factor in patients treated with trimodality therapy. Scheer et al. [27] reported that patients with a pCR after NCRT survived at a rate two times higher than that of other patients. Rohatgi et al. [28] suggested that failure patterns were correlated with the proportion of residual carcinoma after NCRT, implying worse survival of patients who had more residual disease after NCRT.
The clinical complete response is known to be a significant prognostic factor affecting survival, as observed in our previous DCRT studies [4,6]. In this study, 8 of 39 patients showed a clinical complete response immediately after CCRT. The 3-year OS or LRFS rates of patients with a clinical complete response versus non-complete response did not show any statistical significant difference. This would imply that performing esophagectomy after CCRT could have offset an otherwise worse prognosis of patients with a non-clinical complete response, resulting in no different survival outcomes between patients with clinical complete response and those without. According to the published literature, non-responders to NCRT or patients with residual disease after DCRT could survive longer if R0 resection could be achieved [19,20,21]. However, our study showed a large discrepancy in the response rates between clinical response and surgical pathologic response. Only 3 of 8 patients with a clinical complete response showed a pCR (37.5%), and 14 of 31 patients with a non-clinical complete response achieved a pCR (45.2%). Courrech Staal et al. [26] performed a systematic review of several studies comprising 3640 patients treated with NCRT, and they reported that the clinical response was correlated with pathological response in only three studies of 38 studies, and the correlation varied widely (47%-92%). In the evaluation of the clinical response after CCRT, usually endoscopy with biopsy, chest CT, or PET/CT was performed. Stiekema et al. [29] argued that the accuracy of predicting a complete or major pathologic response was limited and did not support the use of FDG-PET/CT for refraining from surgical treatment. Another study also showed that post-CCRT FDG-PET could not rule out residual microscopic disease even if the results of post-CCRT imaging modalities were normal [30]. Alfieri et al. [31] reported that the changes in tumor volume as calculated by using CT scans had a limited role in predicting the pathological response to neoadjuvant treatment in esophageal cancer patients. Furthermore, the use of endoscopic biopsy to determine the entire tumor response after CCRT is debatable. Indeed, Schneider et al. [32] argued that the diagnostic accuracy of endoscopy, rebiopsy, and EUS was inadequate for an objective response evaluation after NCRT, and that only histomorphologic regression was an objective response parameter of significant prognostic importance.
Our study showed that the 30-day postoperative mortality rate was 10.3% (4/39) in the entire patient cohort. In their systematic review of 3,640 patients treated with NCRT, Courrech Staal et al. [26] reported that the in-hospital mortality rate after esophagectomy following NCRT was 5.2% but those NCRT regimens and pathology types varied widely across all analyzed papers. Steyerberg et al. [33] reported that neoadjuvant treatment remained associated with an increased risk of surgical mortality on multivariable logistic regression analysis of the 1,317 SEER 91-96 patients, with adjusted odds ratios (OR) of 2.5 and 1.9 for RT and chemoradiotherapy, respectively. They further reported that comorbidity and age were also highly predictive of surgical mortality, with an OR of 1.6 per comorbid condition, and an OR of 1.6 per decade of age. Moreover, higher-volume hospitals exhibited approximately half the mortality of lower-volume hospitals. Kumagai et al. [34] conducted a meta-analysis of postoperative morbidity in patients receiving NCRT, and reported that NCRT did not increase the risk of postoperative morbidity compared with surgery alone. However, they added that care should be taken for patients with esophageal squamous cell carcinoma, because the risk factors for squamous cell carcinoma, such as tobacco and alcohol use, might make these patients prone to cardiopulmonary damage resulting from a multimodal approach. Although we did not investigate the correlation between tobacco and/or alcohol history with surgical mortality in all patients, the relatively high mortality rate in our patients might be related to the heavy use of tobacco and/or alcohol, both of which are commonly observed in patients with esophageal squamous cell carcinoma in Korea. Chiu et al. [35] tried to determine the optimal interval between NCRT and surgery for esophageal squamous cell carcinoma, and reported that the amount of residual cancer increased significantly after a longer surgical interval, and thereby decreased the survival, probably due to tumor repopulation. Although, we could not find a statistical significant difference of postoperative mortality rates between patients with longer and shorter interval than 8 weeks, there was a little higher tendency of postoperative mortality in patients with longer intervals.
Our study has some limitations. First, this was a retrospective analysis of patients who were treated during a relatively long study period of 11 years; heterogeneous treatment policies in surgery, chemotherapy, and RT might have been applied throughout this period. Most patients (26/39) had received NCRT during the years from 2010 to 2012. However there were no significant differences of treatment, response, and survival outcomes between two study periods of 2002-2009 and 2010-2012, albeit small cohort size (data not shown). Second, this study analyzed a patient cohort with a small number of patients, making it difficult to evaluate the exact role of NCRT plus surgery. Future prospective studies regarding a planned NCRT plus surgery should involve a more consistent treatment policy across a homogenous patient cohort.
In conclusion, our data indicate that only pathological stage was an independent prognostic factor for both OS and LRFS in patients with esophageal cancer treated with esophagectomy after NCRT. We could confirm the significant role of NCRT in downstaging the initial tumor bulk and thus resulting in better survival of patients who gained earlier pathological stage after NCRT.
Acknowledgments
We greatly thank professor Sun-Seog Kweon (Department of Preventive Medicine, Chonnam National University Medical School) for kindly providing his comments for statistical consultation.
Notes
Conflict of Interest: No conflict of interest relevant to this article was reported.