Patterns of recurrence after radiation therapy for high-risk neuroblastoma
Article information
Abstract
Purpose
To investigate the patterns of recurrence in patients with neuroblastoma treated with radiation therapy to the primary tumor site.
Materials and Methods
We retrospectively analyzed patients with high-risk neuroblastoma managed with definitive treatment with radiation therapy to the primary tumor site between January 2003 and June 2017. These patients underwent three-dimensional conformal radiation therapy or intensity-modulated radiation therapy. A total of 14–36 Gy was delivered to the planning target volume, which included the primary tumor bed and the selected metastatic site. The disease stage was determined according to the International Neuroblastoma Staging System (INSS). We evaluated the recurrence pattern (i.e., local or systemic), progression-free survival, and overall survival.
Results
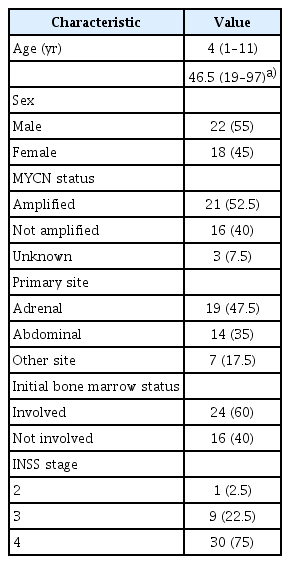
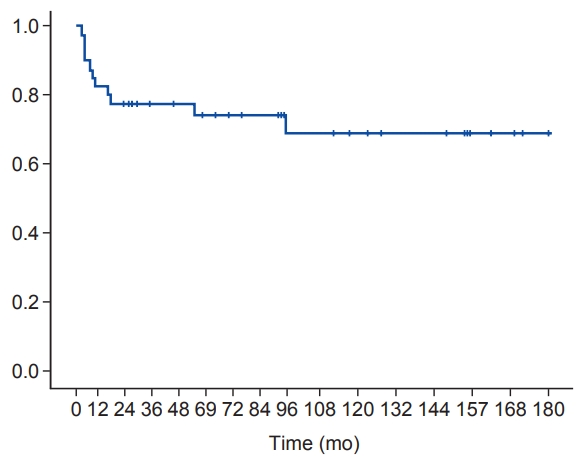
A total of 40 patients with high-risk neuroblastoma were included in this study. The median patient age was 4 years (range, 1 to 11 years). Thirty patients (75%) had INSS stage 4 neuroblastoma. At the median follow-up of 58 months, there were 6 cases of local recurrence and 10 cases of systemic recurrence. Among the 6 local failure cases, 4 relapsed adjacent to the radiation field. The other 2 relapsed in the radiation field (i.e., para-aortic and retroperitoneal areas). The main sites of distant metastasis were the bone, lymph nodes, and bone marrow. The 5-year progression-free survival was 70.9% and the 5-year overall survival was 74.3%.
Conclusion
Radiation therapy directed at the primary tumor site provides good local control. It seems to be adequate for disease control in patients with high-risk neuroblastoma after chemotherapy and surgical resection.
Introduction
Neuroblastoma is the second most common solid tumor in childhood and is the most common extracranial solid tumor in children. Approximately 90% of cases occur in children <10 years of age, and this disease accounts for about 15% of the overall childhood cancer mortality [1-3].
Patients with high-risk neuroblastoma, which require multimodality treatment, account for approximately 50% of all patients with neuroblastoma. These patients are treated with chemotherapy, surgical resection, autologous stem-cell transplantation, and radiation therapy (RT). However, the treatment results of these patients have not been satisfactory.
RT is usually applied to the primary tumor site or surgical bed after the patient has recovered from stem-cell transplantation. Several studies have shown that RT to the primary tumor site improves the local control rate [4-10].
At the time of the initial diagnosis, approximately 50% of patients have metastatic disease and distant recurrence is a major obstacle to the treatment of these patients. However, the patterns of recurrence have rarely been reported. Therefore, we investigated the patterns of recurrence in patients with neuroblastoma treated with RT to the primary tumor site.
Materials and Methods
We retrospectively analyzed patients with high-risk neuroblastoma managed with definitive treatment (i.e., chemotherapy, surgical resection, or stem-cell transplantation) with RT to the primary tumor site between January 2003 and June 2017.
High-risk neuroblastoma was defined according to the Children’s Oncology Group risk stratification system [11]. The disease stage was determined using the International Neuroblastoma Staging System (INSS). Computed tomography (CT), whole-body magnetic resonance imaging (WB-MRI; n = 39, 97.5%), iodine-123 (123I) meta-iodobenzylguanidine single-photon emission computed tomography (MIBG SPECT; n = 27, 67.5%), bone scan, bone marrow aspiration, and biopsy were performed for staging.
Most of the patients underwent chemotherapy according to the COGANBL00P1 study [12] or the CCG3891 study [13]. The treatment response of the patients was evaluated using WB-MRI, CT, bone scan, 123I-MIBG SPECT, and bone marrow aspiration.
After induction chemotherapy, surgical resection, and stem-cell transplantation, RT was delivered to the primary tumor sites or residual tumor. In some patients, RT was concurrently administered to the metastatic site. Patients who received RT with a palliative intent and those who did not receive RT to the primary tumor site were excluded from the current study. The gross target volume consisted of the post-induction chemotherapy, pre-surgical tumor volume. The clinical target volume consisted of the primary tumor bed and regional lymph nodes. The planning target volume was set by expanding the clinical target volume by 0.5–1 cm. The treatment of metastatic disease was different for each patient; however, we usually included the gross tumor and the surrounding 1–2 cm (Fig. 1). A CT-based planning was used for treatment planning. The patients were treated with three-dimensional conformal RT or intensity-modulated RT. A total of 14–36 Gy in 7–20 fractions was delivered to the planning target volume, which included the primary tumor bed and the selected metastatic site (the same dose of radiation was delivered to the primary site and the metastatic site).
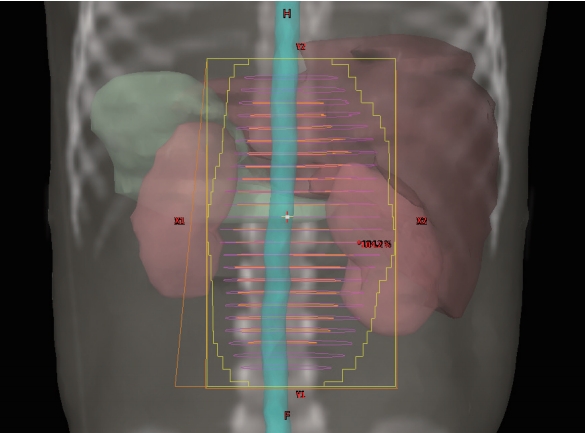
Radiation therapy treatment field for a patient with a left adrenal primary neuroblastoma who underwent left adrenalectomy.
We evaluated the recurrence pattern (i.e., local or systemic), progression-free survival (PFS), and overall survival (OS). Local recurrence was defined as disease recurrence in the primary tumor site or regional lymph nodes. Further, we defined adjacent-field failure as recurrence or progression of disease at sites outside the planning target volume that received <95% and >5% of the prescribed dose. Local failure included both adjacent-field failure and in-field recurrence. PFS was defined as the time from the initiation of RT to disease recurrence or progression or death of any cause. OS was defined as the time from the initiation of RT to the date of the last follow-up or the date of death. SPSS software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the Kaplan-Meier method was used to calculate the PFS and OS.
Results
From March 2003 to June 2017, 40 patients (22 male, 18 female) with high-risk neuroblastoma treated with RT were enrolled in this study. All patients received RT to the primary tumor site. The median age at the time of RT was 4 years (range, 1 to 11 years). The patient characteristics are listed in Table 1. Most of the patients had INSS stage 4 neuroblastoma (n = 30, 75%). The primary tumor sites were mainly in the abdomen (i.e., 19 adrenal and 14 abdominal). MYCN amplification was observed in 21 patients (52.5%).
The treatment characteristics are summarized in Table 2. Thirty-six patients underwent gross total resection (GTR) and 4 patients underwent subtotal resection. After surgery, 35 patients underwent stem-cell transplantation. A total of 40 patients received RT to the primary tumor site. The median radiation dose was 21 Gy (range, 14 to 36 Gy). The most commonly used RT regimen was 21 Gy at 1.5 Gy per fraction two times per day (26 patients). In 8 patients, RT was delivered to the primary site and the initial metastatic site at the same time. The decision to treat the metastatic disease was at the discretion of the treating physician. Usually, the decision for administering RT to metastatic sites was based on the presence of persistent disease after induction chemotherapy or if any residual bulky disease remains. At the median follow-up of 58 months (range, 3 to 180 months), there were 12 cases of recurrence. The individual characteristics of patients with relapsed neuroblastoma and the details of the sites of recurrence are shown in Table 3. Most of the relapsed patients had INSS stage 4 neuroblastoma (10 patients). Among the 12 relapsed patients, 5 had both local and distant recurrence. There were 6 local failure cases and, among these, 4 relapsed adjacent to the radiation field. The other 2 relapsed in the radiation field (i.e., para-aortic and retroperitoneal areas). No disease progression was observed at the irradiated metastatic sites. The main sites of distant metastasis were the bone, lymph nodes, and bone marrow. At the time of analysis, 11 patients had already died (2–80 months after RT).
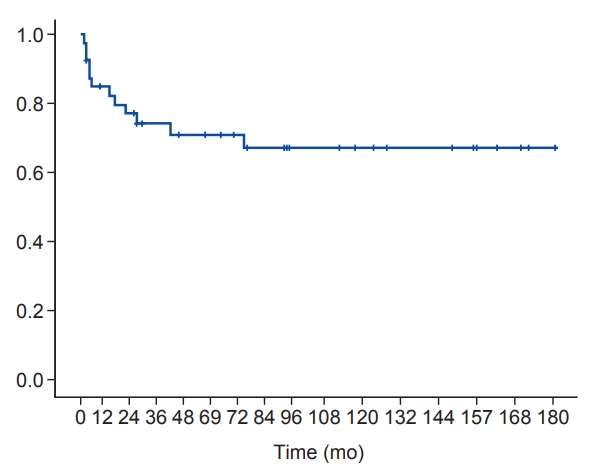
The 5-year OS was 74.3% (Fig. 2) and the 5-year PFS was 70.9 % (Fig. 3). There were only 2 cases (5.0%) of in-field recurrence. OS and PFS tended to be worse in the presence of MYCN amplification or bone marrow involvement. However, there were no statistically significant differences in other results except for OS in patients with MYCN amplification (Figs. 4, 5).

Overall survival (A) and progression-free survival (B) according to the status of bone marrow (BM) involvement.
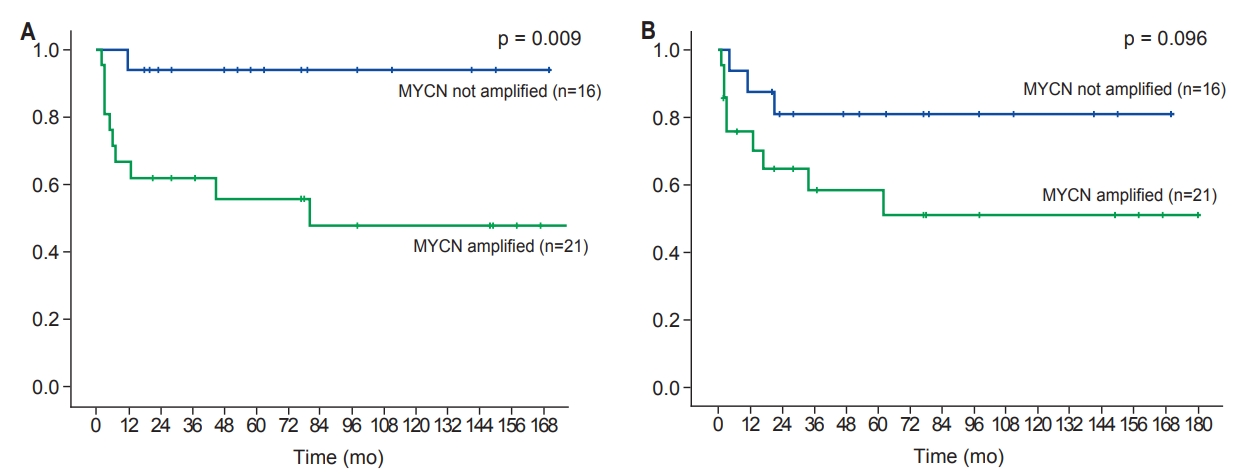
Overall survival (A) and progression-free survival (B) according to the status of MYCN amplification.
Most cases of acute toxicities were grade 1, which mainly included nausea, vomiting, and diarrhea. Severe toxicity of RT (>grade 2) was not observed in the current study, although there was a lack of a detailed description of treatment toxicity in the medical records of our hospital.
Discussion and Conclusion
In patients with high-risk neuroblastoma, local recurrence is the main cause of treatment failure [14,15]. Moreover, surgical resection of the primary tumor combined with systemic therapy improves the local control of the disease and the survival of the patient [15,16]. Several studies have used RT as a consolidative therapy and have reported successful local control [4-10].
One previous randomized controlled trial compared patients who received RT with those who did not [4]. Patients older than 1 year at the time of the diagnosis of Pediatric Oncology Group stage C neuroblastoma were included in this study. After surgery, the patients received RT to the tumor bed/residual tumor and regional lymph nodes. The control group received no RT, and all patients were treated with the same chemotherapy regimen. Patients who received RT showed superior treatment outcomes (event-free survival, 59% vs. 73%; OS, 32% vs. 41%). The results of other studies that showed good local control rates are summarized in Table 4. Bradfield et al. [7] reported a 7% local failure rate at 2 years from RT. RT was delivered to the primary site and to the initial metastatic sites. Gatcombe et al. [10] reported a 3-year local control rate of 94%. RT was delivered to the primary tumor sites regardless of the resection status and metastatic sites according to chemotherapy response. The median RT dose was 22 Gy, and most common fraction scheme used was 21.6 Gy at 1.8 Gy/day. Casey et al. [8] reported that the cumulative incidence of local failure at 5 years after RT (1.5 Gy/fraction, 2 times a day, 21 Gy in total) was 9.8% in patients with high-risk neuroblastoma. In this study, all patients received chemotherapy and underwent GTR. Thereafter, the patients received RT to the primary site. Ferris et al. [9] reported that 67 patients with high-risk neuroblastoma received consolidative RT at a median dose of 21 Gy. RT was delivered to patients with high-risk neuroblastoma as part of definitive treatment. All patients were treated with RT to the primary site, and some of them also received RT to the metastatic sites. The overall local control rate was 92.5%, and no disadvantages were seen with respect to pathologic positive nodes, positive surgical margins, or gross residual disease. Although there is only 1 randomized controlled trial, these data may suggest that RT to the primary tumor site is effective for the local control of high-risk neuroblastoma. Further, considering the results of the current study and the other previously published papers, 21–24 Gy is adequate for local disease control in patients with high-risk neuroblastoma.
Although some studies reported that a higher dose is needed to control gross residual tumor after incomplete surgery [17,18], the optimal dose of RT is still unclear. In this study, 2 patients received boost RT for gross residual tumor with a total radiation dose of 27 and 36 Gy, respectively. Local failure was not observed in these patients. Owing to the small number of patients who received boost RT, it is difficult to conclude the adequate RT dose. However, given that only patients with residual gross tumor had in-field recurrence and no local failure was observed in patients who underwent boost RT, a higher dose may be effective in treating patients with gross residual tumor. It seems necessary to scrutinize the results of ongoing studies.
In our study, most of the patients underwent GTR (90%). The local control rate at the irradiated site was 95%. Only two cases of in-field progression were detected in the current study, and complete resection was not performed in all patients. This result correlates with that of previous reports suggesting the role of RT in improving the local control rate. Given that there is evidence that survival is associated with the local control rate [19], this result suggests that RT plays a vital role in the treatment of high-risk neuroblastoma. Moreover, considering that only patients who had not undergone complete resection experienced recurrence within the RT field, GTR might have an important role in improving local control. This result is consistent with that of a previous study showing favorable outcomes in patients undergoing complete resection [20].
Although metastatic disease is the main cause of death in patients with high-risk neuroblastoma, the role of RT to metastatic sites is still unclear. Some retrospective studies have demonstrated that RT directed to metastatic sites improves the local control at the irradiated metastatic site [7,21-25]. Casey et al. [21] reported the treatment results of 159 patients who received RT to metastatic sites as a consolidative therapy. The 5-year local control rate of the treated metastatic site was 81%. Further, the local control rate was better in the treated sites that were cleared with induction chemotherapy. In our study, 8 patients were treated in both the primary site and in the metastatic sites. Of these patients, 4 experienced disease progression, all of whom had an out-of-field recurrence. In the other 4 patients without recurrence, long-term survival was achieved (98–167 months). There was no case of disease progression within the RT field. Currently, data supporting the efficacy of RT to the metastatic site are insufficient; however, considering that recurrence is common in previously involved sites in patients with neuroblastoma [13,23,24], RT to metastatic sites may improve the tumor control and may be effective in preventing disease progression and prolonging the long-term disease control. RT to the metastatic sites may therefore be beneficial in the treatment of patients with distant tumor involvement. Additional studies are needed to clarify whether RT to metastatic sites is beneficial.
In the older treatment protocol, total body irradiation (TBI) was used as part of a consolidative treatment with chemotherapy and bone marrow transplantation [13,26]. Although TBI was excluded from the treatment protocol because of its long-term toxicity, some articles reported the efficacy of TBI [22,25]. In our study, 2 patients received TBI. It is important to analyze the treatment outcome according to TBI; however, it is difficult to evaluate the role of TBI because of the small number of patients who received TBI in our patient cohort.
The current study has several limitations, including its retrospective nature. First, the composition of the enrolled patients is not uniform. Most of the patients enrolled in this study had INSS stage 4 neuroblastoma with distant involvements. Although these patients are included in a single group (stage 4), they may have different prognoses based on the location of the metastatic disease. A further limitation is the heterogeneity of the chemotherapy protocols. Most of the chemotherapy regimens were based on COGANBL00P1 and CCG3891, although other protocols were included in the patient cohort, with diverse differences in the chemotherapy regimen for patients with recurrence. In addition, only a small number of patients were analyzed, thus leading to insufficient statistical power. However, owing to the rarity of the disease, it would be difficult to conduct a large study. The current study presents the treatment outcome of RT in patients with high-risk neuroblastoma in a single hospital, where the treatment was provided by the same medical team using a relatively homogeneous protocol.
In conclusion, RT seems to be adequate for disease control in patients with high-risk neuroblastoma. RT directed to the primary tumor site provides good local control. However, considering that metastatic disease is an important factor that worsens the prognosis of patients with high-risk neuroblastoma, preventing distant recurrence is essential to improve the treatment outcome. It may be effective to irradiate the metastatic sites; however, supporting evidence is still lacking. Therefore, additional studies are needed to clarify whether RT to metastatic sites is adequate for treating patients with high-risk neuroblastoma.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.