Concurrent chemoradiotherapy improves survival outcome in muscle-invasive bladder cancer
Article information
Abstract
Purpose
To evaluate survival rates and prognostic factors related to treatment outcomes after bladder preserving therapy including transurethral resection of bladder tumor, radiotherapy (RT) with or without concurrent chemotherapy in bladder cancer with a curative intent.
Materials and Methods
We retrospectively studied 50 bladder cancer patients treated with bladder-preserving therapy at Keimyung University Dongsan Medical Center from January 1999 to December 2010. Age ranged from 46 to 89 years (median, 71.5 years). Bladder cancer was the American Joint Committee on Cancer (AJCC) stage II, III, and IV in 9, 27, and 14 patients, respectively. Thirty patients were treated with concurrent chemoradiotherapy (CCRT) and 20 patients with RT alone. Nine patients received chemotherapy prior to CCRT or RT alone. Radiation was delivered with a four-field box technique (median, 63 Gy; range, 48.6 to 70.2 Gy). The follow-up periods ranged from 2 to 169 months (median, 34 months).
Results
Thirty patients (60%) showed complete response and 13 (26%) a partial response. All patients could have their own bladder preserved. Five-year overall survival (OS) rate was 37.2%, and the 5-year disease-free survival (DFS) rate was 30.2%. In multivariate analysis, tumor grade and CCRT were statistically significant in OS.
Conclusion
Tumor grade was a significant prognostic factor related to OS. CCRT is also considered to improve survival outcomes. Further multi-institutional studies are needed to elucidate the impact of RT in bladder cancer.
Introduction
Muscle-invasive bladder cancer (MIBC) constitutes about 30% of newly diagnosed bladder cancers, with about 70% being non-invasive. About 15% of non-invasive bladder cancer cases progress to invasive cancer after transurethral resection of bladder tumor (TURBT) [12].
Radical cystectomy has been a curative treatment option for MIBC. However, mortality and morbidity rates after surgery cannot be ignored [3]. For many patients who are unfit for surgery due to old age, poor medical condition, or patient refusal, radiotherapy (RT) is a good alternative therapeutic option. Surgery produces a 5-year local control rate of about 50% and overall survival (OS) rates of 20% to 40% [45678]. More recently, bladder-preserving therapy including maximal TURBT followed by RT with or without concurrent chemotherapy in selected patients has become widely used.
Bladder-preserving therapy has several benefits compared to radical surgery. Patients with bladder-preserving therapy can have a better quality of life with intact bladder function [2]. In several recent studies, survival outcomes after bladder-preserving therapy are reportedly comparable to those seen in radical cystectomy series [91011121314].
The present study was designed to retrospectively examine survival rates and to evaluate prognostic factors related to treatment outcomes after bladder-preserving therapy with TURBT, RT, and chemotherapy.
Materials and Methods
We analyzed 50 patients with biopsy-proven, the American Joint Committee on Cancer (AJCC) stage II-IV bladder cancer who underwent bladder-preserving therapy at Keimyung University Dongsan Medical Center from January 1999 to December 2010. In the same period, there were 572 patients who were diagnosed to have bladder cancer in our institution, regardless of AJCC stage. The study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center, and informed consent was waived.
Patients underwent physical examinations, complete blood count (CBC), liver function tests, urinalysis, chest radiography, cystoscopy with biopsy, and pelvic magnetic resonance imaging (MRI) or computed tomography (CT) before treatments. Patient performance status was evaluated according to the guidelines of the Eastern Cooperative Oncology Group (ECOG) [15]. During RT, CBC was checked at least once a week. When the absolute neutrophil count was <1,000/mm3 or the platelet count <50,000/mm3, treatments were interrupted or delayed until the patient's condition recovered. Red blood cell transfusion was given to patients with hemoglobin levels below 10.0 g/dL.
TURBT was performed to get accurate diagnosis and maximal tumor removal. If needed, repeated TURBT was also done during follow-up in some patients with suspicion of recurrence. Most underwent TURBT under spinal anesthesia.
External beam RT (EBRT) was delivered using 6, 10, 15, or 20 MV photon beams with a four-field box technique to a dose of 63 Gy in 35 fractions for 5 days per week within 7 weeks. To define the initial pelvic fields in most of the patients, the superior border was at the middle of the sacroiliac joint or at the L5-S1 interspace and the interior border was at or just below the bottom of the obturator foramen. The inferior border was sometimes extended to the bottom of the ischial tuberosity, depending on disease involvement with the prostatic urethra or bladder neck. The lateral border was 1.5 cm lateral to the true pelvis to encompass the bladder and the pelvic lymph nodes. On the lateral portal, the anterior border was placed in front of the bladder and the posterior border was set with at least a 3-cm margin behind the posterior border bladder wall. The portals were reduced after 45 Gy (1.8 Gy per fraction) with a follow-up imaging study using a CT scan and the boost treatment included an initial gross tumor volume with 2-cm margin.
Concurrent chemotherapy was administered every week by intravenous infusion. Cisplatin (40 mg/m2) was given on the first day of the chemotherapy cycle (D1, D8, D15, and D22) within 16 hours after RT.
After completion of treatment, all patients were evaluated by radiation oncologists and urologic oncologist at 3-month intervals for 1 year, and at least 6 months thereafter. Complete response (CR) was defined as no evidence of gross tumor on follow-up cystoscopic examination, and pelvic MRI or CT 3 months after completion of treatment. Partial response (PR) was defined as >30% reduction of gross tumor volume compared to the initial tumor [16].
Local recurrence was defined as any disease recurrence lesions within pelvic radiation fields that had developed 3 months later, after confirmation of CR to treatment. Distant metastasis was also defined as any disease outside the pelvic radiation fields.
OS time was calculated from the time of diagnosis to the date of death or last follow-up. Disease-free survival (DFS) was calculated from the end of primary treatment to the date of diagnosing locoregional recurrence or distant metastasis in patients with CR after RT. Kaplan-Meier analysis was used to estimate the OS and DFS rates, with the log-rank test for prognostic significance, and Cox proportional hazards model for multivariate analysis. A chi-square test was used to analyze the relation between factors in the two groups. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM, Armonk, NY, USA).
Results
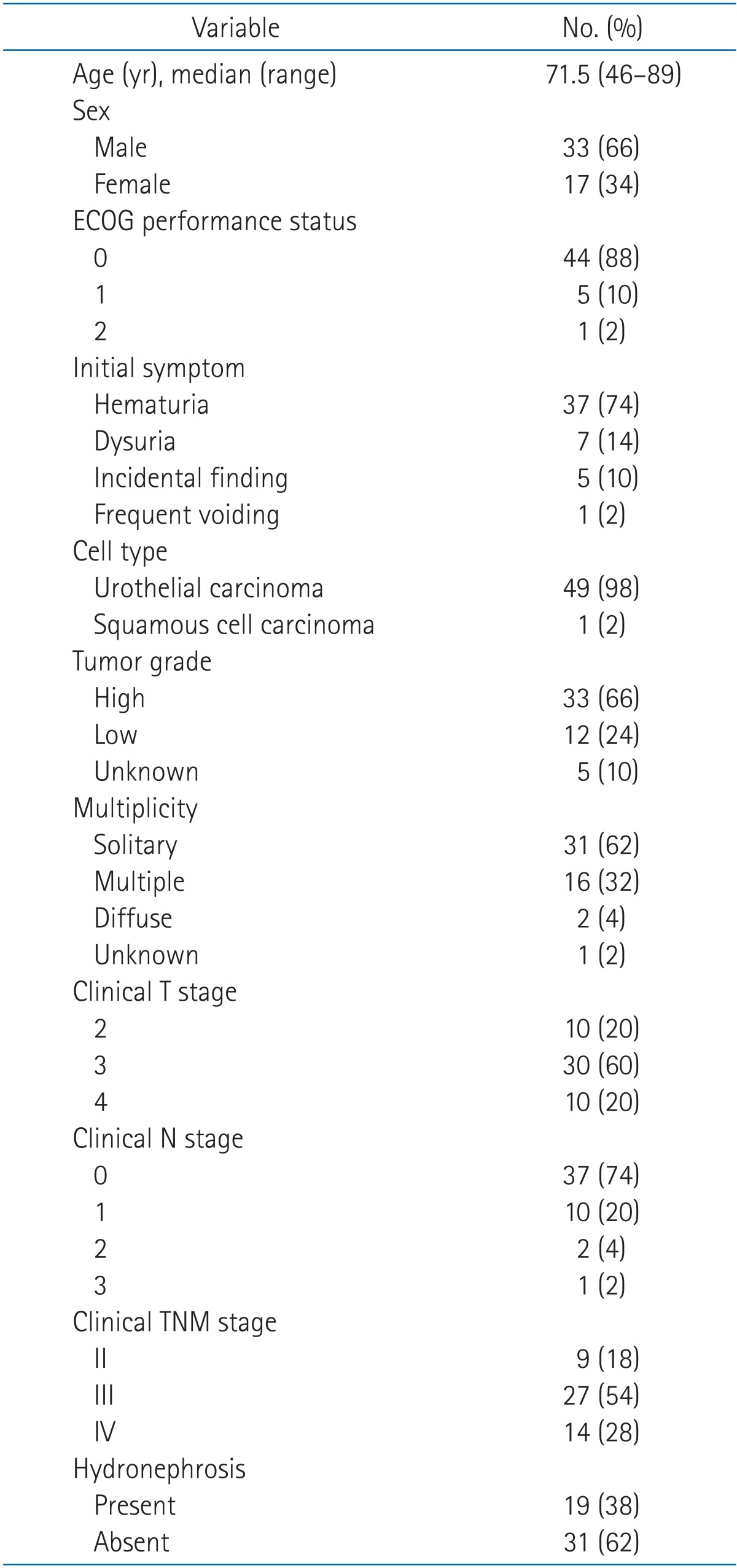
The pretreatment characteristics of the 50 patients are listed in Table 1. Patient age ranged from 46 to 89 years with a median of 71.5 years. Gross hematuria was the most common initial symptom in 37 patients. Seven patients suffered from dysuria, one patient complained increased urinary frequency, and the rest five patients were diagnosed incidentally. Patients had several underlying diseases, such as angina pectoris or acute myocardial infarction in five patients, hypertension in 15, and diabetes mellitus in nine. Fourteen patients had personal history of cigarette smoking.
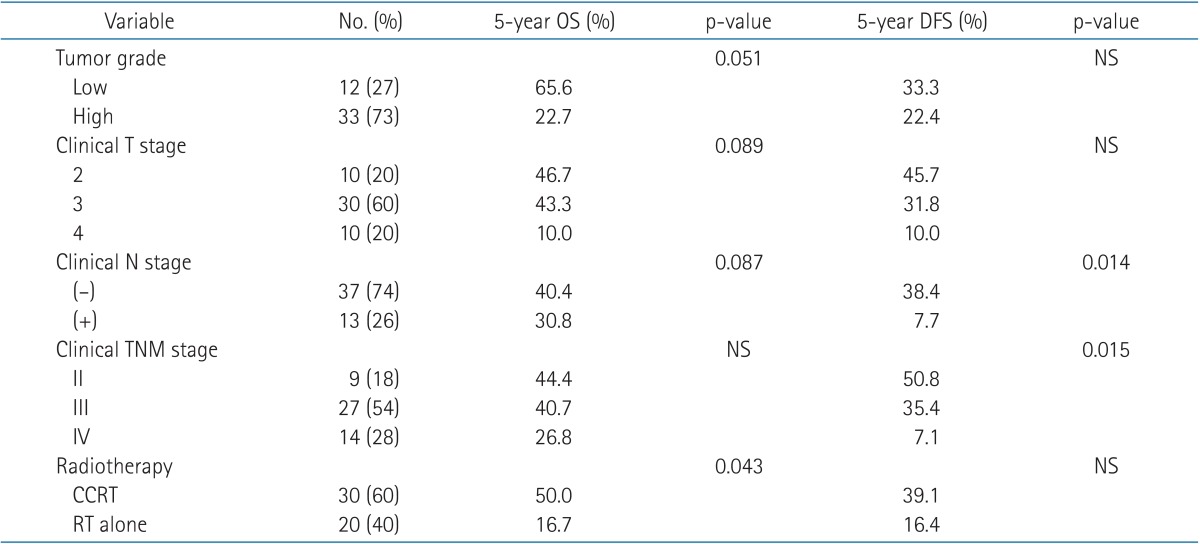
Most patients showed good performance status of ECOG 0 or 1. On pathologic examination, 49 patients (98%) had urothelial carcinoma and one (2%) had squamous cell carcinoma. About two-thirds (33 patients) had high grade tumors. AJCC stage was II in nine patients (18%), III in 27 patients (54%), and IV in 14 patients (28%). Thirty-one patients (62%) showed solitary tumor in bladder, 16 patients (32%) had multiple tumors, and two patients (4%) with diffuse appearing tumor in CT scan. Hydronephrosis was reported in 19 patients (38%) and 31 patients (62%) had normal kidneys. Initial hemoglobin level ranged from 8.6 to 14.9 g/dL with a median of 11.15 g/dL.
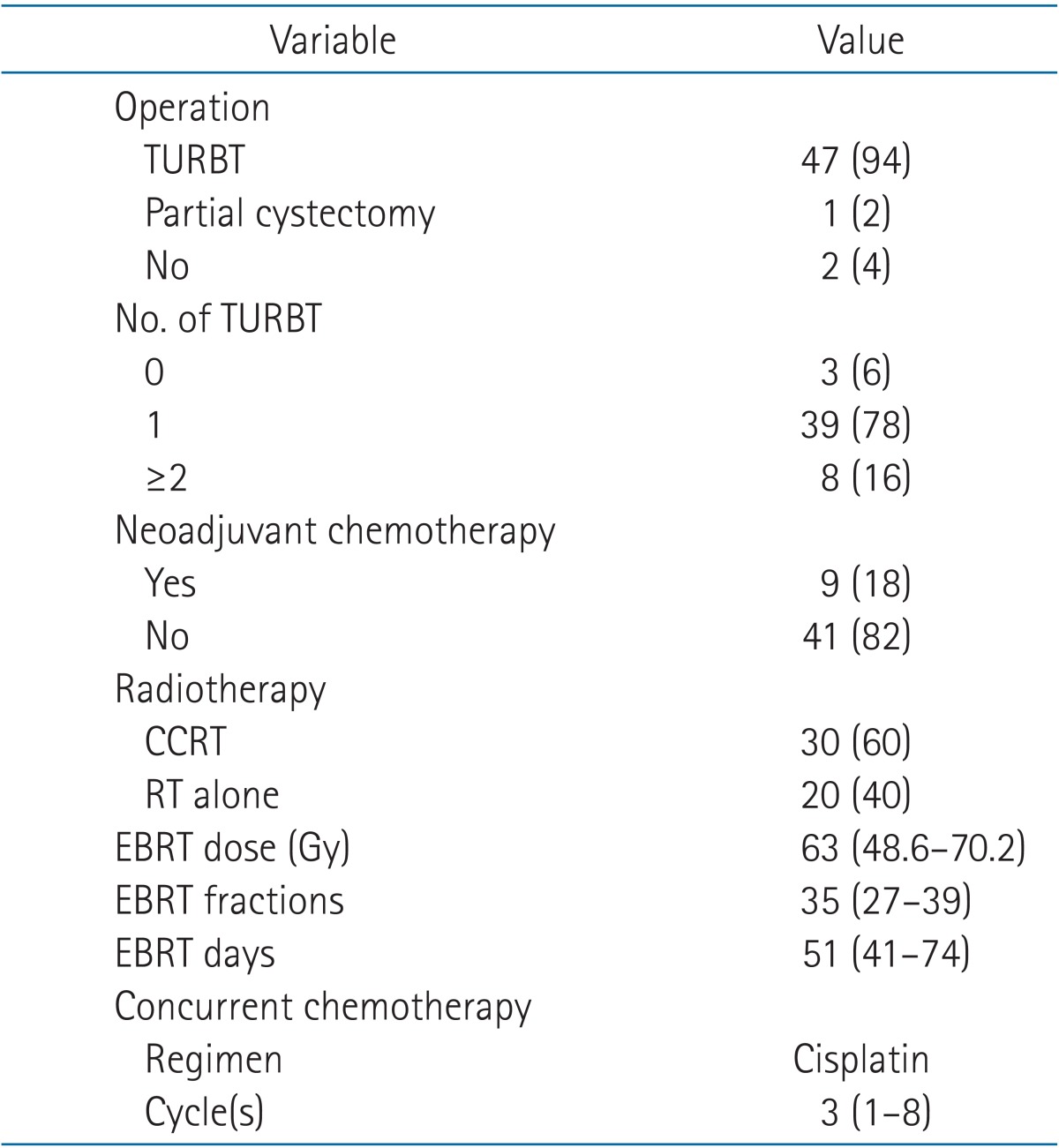
Treatment details are summarized in Table 2. Forty-seven patients (94%) underwent TURBT for accurate pathologic diagnosis and maximal tumor eradication, and one patient (2%) had partial cystectomy. The remaining two patients were diagnosed using cystoscopic examination and cytology due to their poor medical conditions. Of the 47 patients with TURBT, 39 patients underwent TURBT once, six patients did twice. The remaining two patients continued repeated TURBT, three times and five times, respectively. Complete TURBT was done in 11 patients and incomplete TURBT in 36 patients (Table 3).
After pathologic diagnosis, nine patients (18%) had systemic chemotherapy before RT. Chemotherapeutic regimens were GC (gemcitabine and cisplatin) or MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 2 to 10 cycles (median, 8 cycles). Three patients showed PR after systemic chemotherapy, two patients remained in stable disease, and the rest four patients showed disease progression.
Thirty patients (60%) had RT combined with synchronous chemotherapy using cisplatin regimen with 1 to 8 cycles (median, 3 cycles). Other 20 patients (40%) were treated with RT alone. The range of EBRT was 48.6 to 70.2 Gy (median, 63 Gy) and it took 41 to 74 days (median, 51 days) to finish RT.
Three months after completion of treatments, 30 patients (60%) showed CR and 13 (26%) displayed PR. Five patients showed progressed disease and the rest two remained without response to RT. Concerning patient status, 12 patients had no evidence of disease, one were alive with disease, six died from other causes, and 31 died from bladder cancer (Table 3).
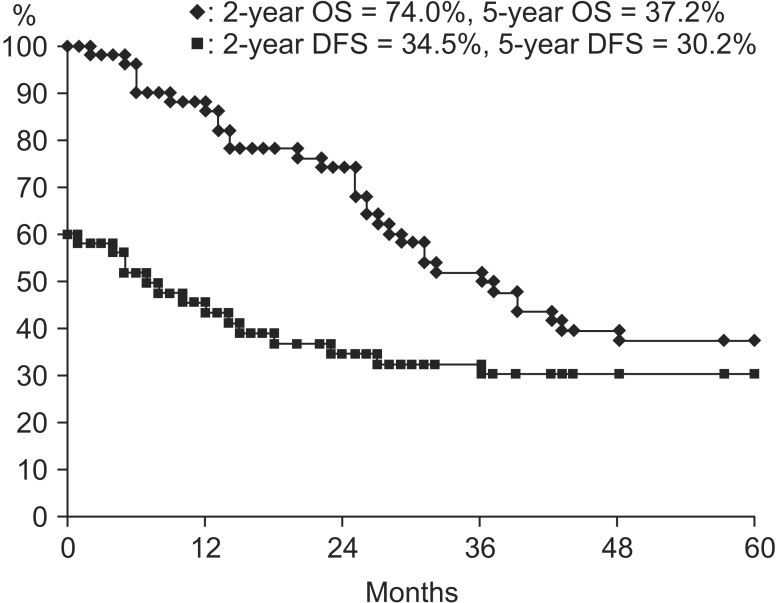
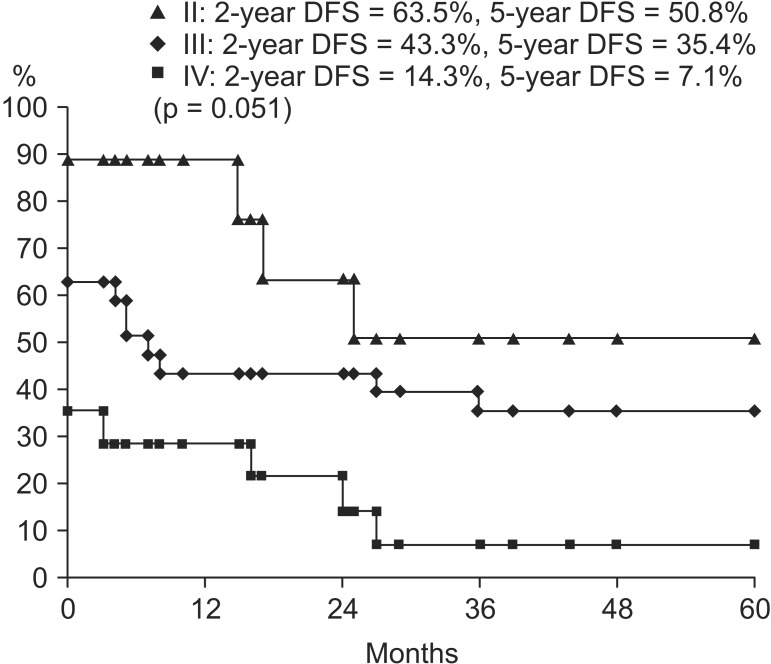
The range of follow-up was 2 to 169 months (median, 34 months) in all patients. Two-year and 5-year OS rate was 74.0% and 37.2%, respectively, and 2-year and 5-year DFS rate was 34.5% and 30.2%, respectively (Fig. 1). During the follow-up period, 10 patients had local recurrence, seven patients had distant metastasis, and two patients had both local recurrence and distant metastasis (Table 3). The specific sites of local recurrence were within the bladder (11 patients) and sigmoid colon (one patient). Of the nine patients with distant metastases, four showed multiple bone metastases including skull and spine, four in the lung, and one in both multiple bones and lung. There was no patient who underwent salvage cystectomy during the follow-up time.
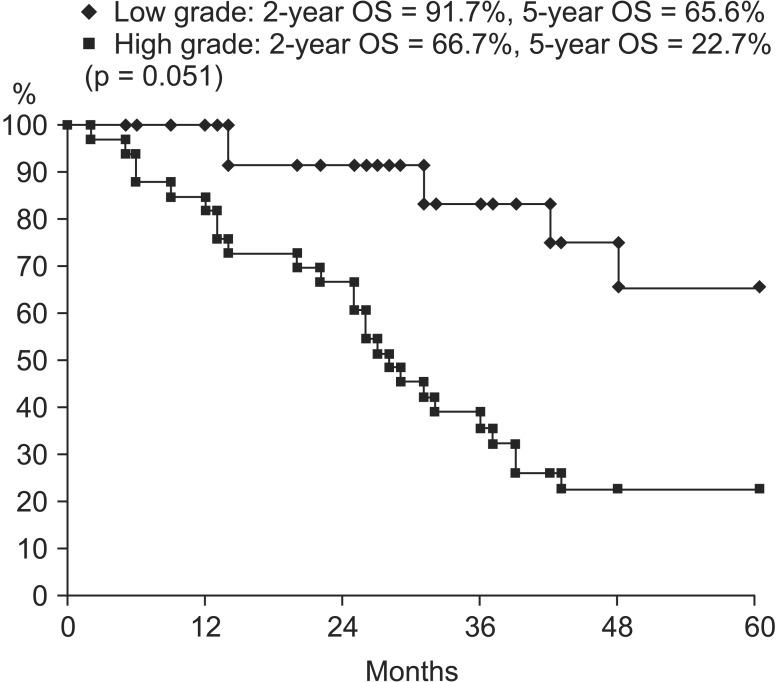
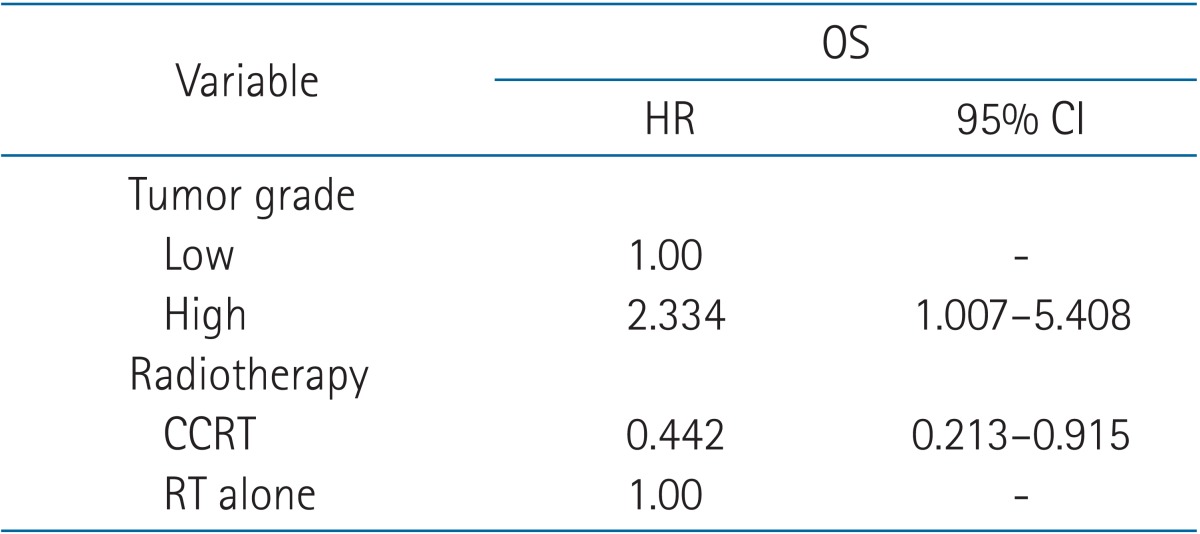
On univariate analysis, concurrent chemotherapy (CCRT vs. RT alone; p = 0.043) was a significant prognostic factor in OS (Fig. 2). Tumor grade (low vs. high; p = 0.051), clinical T stage (cT2 vs. cT3 vs. cT4; p = 0.089), and clinical N stage (cN negative vs. cN positive; p = 0.087) showed marginal significance in OS. OS curves according to tumor grade are in Fig. 3. Clinical N stage (cN negative vs. cN positive; p = 0.014) and clinical TNM stage (II vs. III vs. IV; p = 0.015) were statistically significant prognostic factors in DFS (Fig. 4). These factors are listed in Table 4. Completion of TURBT, number of TURBT, and neoadjuvant chemotherapy were not prognostic factors related to survival rates.

Overall survival (OS) curves according to concurrent chemotherapy. CCRT, concurrent chemotherapy and radiotherapy; RT, radiotherapy.
On multivariate analysis, among the prognostic factors with p ≤ 0.1 on univariate analysis, tumor grade (hazard radio [HR], 2.334; 95% confidence interval [CI], 1.007 to 5.408) and concurrent chemotherapy (HR, 0.442; 95% CI, 0.213 to 0.915) were statistically significant for OS (Table 5). According to the chi-square test, there was no statistically significant difference in the relation between factors such as completion of TURBT, number of TURBT, and clinical stage in the two groups, respectively (low grade vs. high grade, CCRT vs. RT alone).
Discussion and Conclusion
Radical cystectomy still remains the standard curative treatment in MIBC. Recently, bladder-preserving therapy with maximal TURBT, RT, and concurrent chemotherapy has been evolved [2]. Our study was designed to evaluate survival rates and prognostic factors related to treatment outcomes after bladder-preserving therapy including RT in MIBC. There were several limitations to this single institutional retrospective study. First, it is not feasible to generalize our results due to the small number of patients. There were only 50 patients selected to be analyzed in spite of relatively long 12-year follow-up period. It was not possible to access the acute and late toxicities caused by RT or chemotherapy, because patient data was collected retrospectively.
Survival rates seemed inferior to other results, although exact comparison is difficult, as other studies assessed treatment outcomes for different distribution of clinical stages [911]. Kotwal et al. [11] analyzed 169 patients and showed the treatment outcomes after radical RT in the form of EBRT or radical cystectomy. About a half of patients were in stage T3 or worse disease in radical RT group and the 5-year OS and disease-specific survival (DSS) was 34.6% and 56.8%, respectively. A recent pooled analysis of RTOG reported 5-year OS of 57% and 5-year DSS of 71% in patients whose clinical stages were T3 or worse in 39% [9]. In our study, 80% of patients were in clinical T3 or T4.
Patterns of failure after treatment were consistent with previously reported results. The majority of locoregional failures were in the bladder, and there were more non-invasive cases in the BC2001 study [17]. Presently, 11 out of 12 patients (91.7%) had bladder recurrence, highlighting the importance of careful follow-up for bladder recurrence after bladder-preserving therapy. We also analyze the effect of concurrent chemotherapy on locoregional recurrence and distant metastasis. Synchronous cisplatin was reported to have a highly significant effect on pelvic recurrence and no effect on distant metastasis, whereas chemotherapy prior to RT had no effect on whether loco-regional recurrence or distant metastasis [1819]. However, concurrent cisplatin was not effective on both locoregional recurrence (p = 0.137) and distant metastasis (p = 0.724) in our study. This may have reflected patient numbers; a larger number of patients may yield a satisfactory and accurate result.
For the evaluation of prognostic factors to survival rates, tumor grade was related to OS. It is already known to be the most important related to patient prognosis [20]. CCRT was another prognostic factor related to OS in multivariate analysis. Currently, although there are several combinations of concurrent or sequential chemotherapy with RT, concurrent chemotherapy with cisplatin is the most common combination to treat MIBC [212223]. We also analyze clinical T stage and clinical N stage as well as clinical TNM stage for prognostic factors, respectively, because clinical T stage represents the depth of tumor invasion by itself and clinical N stage either negative or positive is able to determine clinical TNM stage, even in same clinical T stages.
During the follow-up period, all patients have their own bladder preserved, because no one underwent salvage cystectomy. However, it does not demonstrate successful treatment results. Eleven patients with local recurrences in bladder underwent repeated TURBT or palliative chemotherapy, instead of salvage cystectomy, due to old age or their poor medical conditions such as history of acute myocardial infarction or angina pectoris.
Bladder-preserving therapy in MIBC has several advantages including higher quality of life with comparable survival outcomes to radical cystectomy and is able to be adopted for patients with old age. For the bladder-preserving therapy including TURBT followed by RT, synchronous chemotherapy with RT would improves survival outcome. Tumor grade is also important prognostic factor for better survival outcomes. It is suggested that further prospective or multi-institutional studies are necessary for MIBC.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.