Hypofractionated radiotherapy for early stage glottic cancer: efficacy of 3.5 Gy per fraction
Article information
Abstract
Purpose
The purpose of this study was to evaluate the treatment outcomes and toxicity profile of patients with early glottic cancer who underwent hypofractionated radiation therapy (RT) with 3.5 Gy per fraction.
Materials and Methods
A retrospective review was performed of the medical records of 35 patients with early stage (T1-2N0M0) glottic cancer who underwent definitive RT. The dose fractionation scheme was 59.5 Gy in 17 fractions. Posterior commissure was excluded from the clinical target volume (CTV) for 26 patients (74.3%) without glottic lesions close to this region.
Results
With a median follow-up of 16.23 months (range, 6.82 to 67.15 months), no local, regional, or distant recurrence was reported. Acute hoarseness (65.7%), mucositis (68.6%), radiation dermatitis (60.0%) was frequent. One patient (2.9%) reported grade 3 acute toxicity (mucositis) and there was no grade 4–5 acute toxicity. There was no grade ≥3 late toxicities; however, grade 1 late intermittent hoarseness was frequent (45.7%). The receiver operative characteristic analysis revealed that mean hypopharyngeal dose was predictive for acute grade ≥2 mucositis (area under the curve=0.9314; 95% confidence interval, 0.8524–1). The optimal threshold of mean hypopharyngeal dose for occurrence of acute grade ≥2 mucositis was 26.31 Gy, with a specificity and sensitivity of 83.3% and 88.2%, respectively.
Conclusion
Hypofractionated RT with fraction size of 3.5 Gy for early glottic cancer is effective. The hypopharyngeal mean dose could predict the occurrence of grade ≥2 acute mucositis. The posterior commissure can be safely excluded from the CTV.
Introduction
The mainstay of treatment for early stage (T1-2) glottic laryngeal cancer is radiation therapy (RT) and surgery at the primary site. This is because early glottic cancer rarely metastasizes to regional lymph node or distant organs [1]. RT alone can achieve high local control and laryngeal preservation rates [2,3]. Many institutions have implemented hypofractionated RT regimens using up to 2.5 Gy per fraction and shown good local control with tolerable toxicity [4-6]. Recent technological advances made it possible to further increase the dose per fraction, which can lead to better treatment outcomes and shorter treatment time for various types of cancer [7]. Early glottic cancer is a good candidate for this trend. Some clinicians have insisted that hypofractionated regimen should be standard in RT for T2 glottic cancer [8]. Additionally, there was a clinical trial to increase the fraction size up to 8.5 Gy [9]. Nevertheless, increasing the fraction size should be applied with caution because severe acute and late toxicities may occur [10].
Our group conducted a phase I dose escalation trial for early glottic cancer. However, the trial was terminated due to a high rate of severe toxicities (33.3%), such as vocal cord ulcer and cartilage necrosis in the 5 Gy per fraction arm [11]. Nonetheless, the trial showed that RT with 3.5 Gy per fraction can be safely delivered to the glottis with no report of severe toxicities. Therefore, we implemented this fractionation scheme in our institution. The purpose of this study was to evaluate the treatment outcomes and toxicity profile of patients with early glottic cancer who underwent RT with 3.5 Gy per fraction.
Materials and Methods
1. Study population
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. H-2104-233-1218) before collecting patient information. The informed consent was waived. A retrospective review was performed of the medical records of 35 patients who underwent RT for histologically confirmed early (T1-2N0M0) glottic squamous cell carcinoma with a dose fractionation scheme of 59.5 Gy in 17 fractions from January 2015 to December 2020. Patients with a follow-up period shorter than 6 months from the start of RT were excluded. Seven patients were from the 3.5 Gy per fraction arm of the previous prospective trial in 2015; 28 were treated from 2018–2020.
2. Treatment and follow-up
RT was similarly planned with the previous prospective trial of the institution, as described previously [11,12]. Briefly, patients underwent simulation computerized tomography (CT) scan in supine position with both arms adducted. Thermoplastic Aquaplast was used to immobilize the patient. The patients were instructed not to swallow during simulation CT scan and treatment. The target delineation method has been slightly modified since 2018. Previously, the clinical target volume 1 (CTV1) was defined as the gross tumor volume and the CTV2 was defined as remaining larynx from the thyroid notch to the inferior border of the cricoid cartilage. From 2018, posterior commissure was excluded from the CTV2 unless the lesion was close to it. As a result, posterior commissure was treated in 9 (25.7%) patients (7 from the previous trial). Examples of CTV delineation and dose distribution are illustrated in Fig. 1. The planning target volume (PTV) was constructed by expanding the CTVs by 3 mm. CTV-to-PTV expansion to the posterior direction was reduced when the CTV was close to the hypopharynx. Volumetric modulated arc therapy (VMAT) with simultaneous integrated boost was applied for RT planning and two arcs (180°–230°) of 6 MV photon beam were used. The dose prescription was as follows: 59.5 Gy in 17 fractions to PTV1 and 47.6 Gy in 17 fractions to PTV2. One fraction was delivered each day, 5 days per week. All patients underwent laryngomicrosurgery to obtain tissue for histologic confirmation before RT. Laryngoscopic examination was conducted to assess laryngeal and hypopharyngeal abnormalities during RT course.
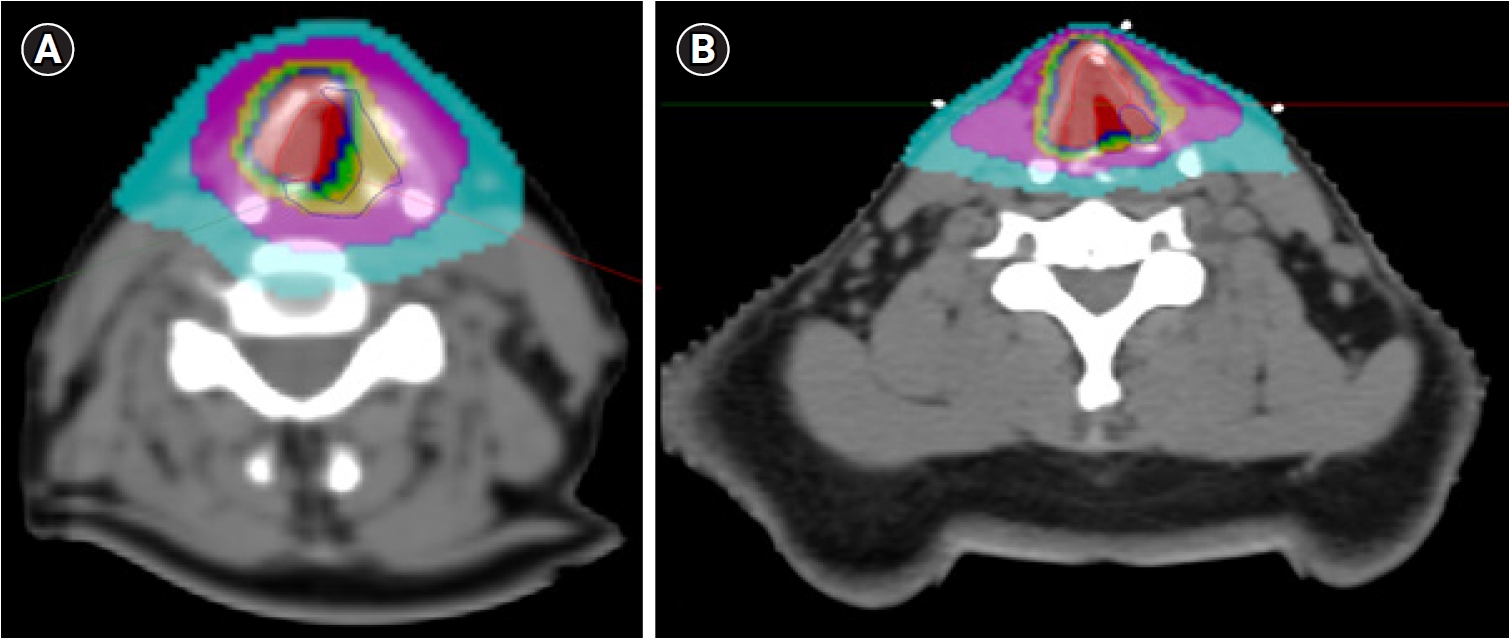
Clinical target volume (CTV) delineation and dose distribution of (A) a patient enrolled in the previous prospective trial and (B) a patient who underwent radiotherapy after modification of the target delineation method. The red and blue lines represent CTV1 (59.5 Gy) and CTV2 (47.6 Gy).
The patients were followed up at 2 weeks, 1, 3, and 6 months, and 1 year after RT completion. Thereafter, the patients were advised to follow up at a 4-month interval in the second year and 6-month interval in the third to fifth years. Clinical examination with laryngoscopic evaluation was mainstay of follow-up modality, and imaging was optional.
3. Clinical outcomes and toxicity profile
The clinical outcomes in this study were local control (LC), regional control (RC), distant control (DC), and overall survival (OS). An LC event was defined as progression of disease in the glottic larynx, while a RC event was defined as metastasis to the neck lymph node area. A DC event was defined as the occurrence of distant metastasis and an OS event was defined as the death of the patients from any cause.
A new occurrence or worsening of the symptoms or signs of hoarseness, mucositis, and radiation dermatitis were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5. Toxicity that occurred during the RT course or <3 months after RT completion was categorized as acute toxicity; other events were categorized as late toxicity. We hypothesized that exclusion of posterior commissure from the CTVs may reduce dose to the hypopharynx and occurrence of mucositis. Therefore, we evaluated hypopharyngeal dose threshold for occurrence of mucositis via receiver operating characteristic (ROC) curve analysis. The hypopharynx was defined from the hyoid bone to cricoid cartilage, and delineated during RT planning or for this retrospective analysis. The ROC curve was created for occurrence of grade ≥2 acute mucositis by mean hypopharyngeal dose. The optimal dose threshold was calculated by finding the point minimizing the Euclidean distance between the ROC curve and the (0,1) point [13]. All statistical analyses were performed using R 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Patient characteristics
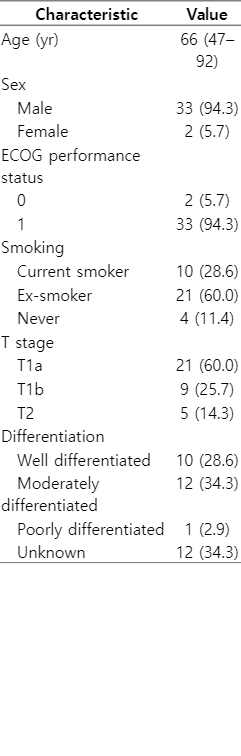
Patient characteristics are summarized in Table 1. The patients were predominantly male (94.3%), and 88.6% had a history of smoking. The median pack-year of smokers was 30 (range, 3 to 80). Six smokers had unknown history of cigarettes consumed. The median interval between base of follow-up and termination of smoking for ex-smokers was 78 months (range, 12 to 360 months), with three ex-smokers with unknown intervals. At the time of diagnosis of glottic cancer, 10 patients were current smokers, and four smokers failed to quit smoking after the diagnosis. Thirty patients (85.7%) had T1 disease. The median overall treatment time was 24 days (range, 22 to 29 days).
2. Treatment outcomes
The median follow-up from the start of RT was 16.23 months (range, 6.82 to 67.15 months). There was no local, regional, or distant recurrence reported. Therefore, the LC, RC, and DC rates were 100%. The crude rate of OS was 97.1%. One patient was died five days after 6-month follow-up visit. He was seventy-four years old. In the last follow-up visit, the patient reported waxing and waning hoarseness and there was residual erythema on the neck. In laryngoscopic examination, no laryngeal mucosal lesion was found and the vocal cord had good mobility. Exact cause of death was unknown.
3. Toxicity profiles
Toxicity profiles of the patients are summarized in Table 2. Acute hoarseness, mucositis and radiation dermatitis was frequent during or within 3 months after completion of the RT course (hoarseness, 65.7%; mucositis, 68.6%; radiation dermatitis, 60.0%), although these acute toxicities were mostly grade 1–2. Only one (2.9%) grade 3 acute toxicity (mucositis) was reported and there was no grade 4–5 toxicity. The patient with grade 3 acute mucositis had odynophagia during the RT course, and revisited the outpatient department 4 days after RT completion due to a worsening of throat pain and odynophagia. Posterior commissure was not included in the CTV for this patient. Hemorrhagic ulceration at the hypopharynx was found during laryngoscopic examination and an opioid analgesic was prescribed. After supportive care without other intervention, the symptoms were spontaneously subsided. No residual mucositis was found in laryngoscopic examination performed in 6 months after RT completion. The patients with posterior commissure included in the CTV had a higher rate of grade ≥2 acute mucositis (88.9% vs. 34.6%, p = 0.015). No treatment interruption or discontinuation due to acute toxicity was reported. In terms of late toxicities, there was no grade ≥3 event reported in the entire cohort. Late hoarseness was frequent (48.6%), but mostly intermittent. Late radiation dermatitis was rare (5.7%). No late mucositis was reported.
4. Mean hypopharyngeal dose and prediction of mucositis
The patients with posterior commissure included in the CTV had a median mean hypopharyngeal dose of 36.20 Gy (range, 27.06 to 45.72 Gy). In contrast, the patients without posterior commissure in the CTV had a median dose of 23.91 Gy (range, 13.03 to 35.66 Gy). This difference was statistically significant (p < 0.001). The patients with acute grade ≥2 mucositis had a median mean hypopharyngeal dose of 32.35 Gy (range, 23.90 to 45.72 Gy), while the patients without acute grade ≥2 mucositis had a median dose of 21.80 Gy (range, 13.03 to 33.27 Gy). This difference was also significant (p < 0.001). In the ROC analysis, mean hypopharyngeal dose was predictive for acute grade ≥2 mucositis (area under the curve=0.9314; 95% confidence interval, 0.8524–1). The ROC curve is illustrated in Fig. 2. The optimal threshold of mean hypopharyngeal dose for occurrence of acute grade ≥2 mucositis was 26.31 Gy, with a specificity and sensitivity of 83.3% and 88.2%, respectively. Among 18 patients with mean hypopharyngeal dose higher than 26.31 Gy, 15 patients (83.3%) had acute grade ≥2 mucositis. Two patients (11.8%) from remaining 17 patients with mean hypopharyngeal dose less than 26.31 Gy had acute grade ≥2 mucositis.
Discussion and Conclusion
The previous prospective trial from our group reported that definitive RT with a dose fractionation scheme of 59.5 Gy in 17 fractions for early glottic cancer had no dose-limiting toxicities or recurrence. The current study provided additional evidence with a larger cohort who underwent definitive RT for early glottic cancer with a fraction size of 3.5 Gy. All patients included in this study were disease-free during the follow-up period, and low rates of severe acute and late toxicities were reported.
Hypofractionation may lead to better treatment outcomes for slowly growing tumors, and it has become the standard treatment method for breast and prostate cancer [14]. Many studies have evaluated hypofractionated RT for early glottic cancer with fraction sizes up to 2.5 Gy, and resulted in better local control or survival rates [4,6,15]. Furthermore, there was an effort to apply 52.5 Gy in 3.28 Gy per fraction to early glottic cancer, with a 5-year LC rate of 93% and 82% for T1 and T2 tumors, respectively, which is comparable to other fractionation regimens [8,16]. It should be noted that the fractionation scheme of the current study has relatively high biologically effective dose (BED). BED of dose-fractionation schemes from the current study and other previous studies calculated by the equation based on linear quadratic model with consideration of tumor proliferation are summarized in Table 3 [4,9,11,17,18]. We applied the same equation and parameters with Lim et al. [3]: 15 for α/β ratio, 0.35 for α, 28 days for kick-off time, and 3 days for potential doubling time. Furthermore, in the current study, this dose was delivered over a median treatment time of 24 days. Tumor clonogen repopulation during RT can start at 4 weeks after the start of RT [19]; therefore, irradiating a sufficient dose in a short period of time may increase the tumor control probability. No recurrence was reported in the current study with a median follow-up of 16.23 months, which may indicate that this fractionation scheme is at least comparable to the previous dose-fractionation schemes in terms of local control. However, further follow-up is required.
One of the concerns about applying hypofractionated RT is the potential of increased toxicity. Some studies with moderate hypofractionation with fraction size of 2.2–2.5 Gy reported higher incidences of acute toxicity [20,21]. The crude rates of occurrence of acute hoarseness, mucositis, and radiation dermatitis in the current study were high; however, all reported acute toxicities were mild except one case of grade 3 mucositis. It should be noted that wide range of mild to moderate acute toxicity rates have been reported due to different methodology or criteria. For instance, Moon et al. [15] reported 9% of grade 1 mucous membrane toxicity and 21% of grade 1 larynx toxicity in hypofractionation arm with no grade 2 acute mucous membrane and larynx toxicity, while Kodaira et al. [22] reported 80.3% of grade 1–2 dysphagia and 95.1% of grade 1–2 voice change in hypofractionation arm. The crude rate of grade 2 mucositis in the current study was 45.7%; nonetheless, not all RT plans used in the current study applied hypopharyngeal dose limitation. We proposed a threshold of hypopharyngeal mean dose for mucositis, which means that the appropriate dose limitation to the hypopharynx may decrease the occurrence and severity of acute mucositis. The dose-response relationship between radiation dose and mucositis have been widely studied; reports and examinations for oral mucositis showed that mild or minimal mucositis is expected under approximately 30 Gy, whereas severe changes, such as ulceration, appear with accumulated doses >30 Gy [23,24]. The optimal threshold of hypopharyngeal dose suggested in the current study was 26.31 Gy. BED with α/β ratio of 10 (without consideration of time factor) for this dose is 30.38 Gy, which is close to BED for 28 Gy in 30–35 fractions. Note that fraction numbers of 30–35 were frequently used in conventional fractionation schemes in head and neck cancer. This dose threshold is slightly lower than previous studies, which may be due to different toxicity criteria, endpoints, treatment times, or anatomy. In addition, potential of increased severe long-term laryngeal complications such as cartilage necrosis is needed to be considered for applying hypofractionated RT. In the current study, low rates of severe late toxicities were reported, but longer follow-up would be warranted for addressing this issue adequately.
Conventional treatment portals for early glottic cancer include the thyroid notch superiorly to cricoid cartilage inferiorly with the posterior border adjusted by the location of the lesion [25]. In the previous prospective trial from our group, the CTV delineation principle was partly based on this conventional treatment portal settings. The whole larynx, from the thyroid notch to the cricoid cartilage, was included in the low-dose CTV. Using the simultaneous integrated boost technique, higher dose could be delivered to the glottic lesion without extending overall treatment time. This CTV delineation principle was adjusted based on the evidence that posterior commissure is less-frequently involved by glottic squamous cell carcinoma [26]. By excluding posterior commissure from the CTV, the hypopharyngeal dose can be controlled more easily, and this may lower the severity and occurrence of acute mucositis, as hypothesized from the proposed dose threshold of the current study. Rather than treating the whole larynx, inclusion of high-risk area only to the CTV considering location of the glottic lesion could be also feasible. Target volume delineation principle of Sher et al. [9] is a good example. A more radical reduction of the target volume has been made in previous literature. Al-Mamgani et al. [27] have reported irradiating involved vocal cord only with a hypofractionated regimen of 58.08 Gy in 16 fractions for T1a glottic cancer. With a median follow-up of 30 months, they reported 2-year LC of 100%. In the current study, 40.0% of the included patients had T1b-2 tumors, and no recurrence was reported. For T1b-2 tumors, our principles of CTV delineation may be more suitable, although adjustment of the principle would be needed. We are considering inclusion of the larynx within 1 cm from the glottis superiorly and inferiorly to the CTV, rather than including the whole larynx. Even though reducing target volume may be beneficial for toxicity profile, caution should be taken when applying new principle due to microscopic tumor extension and nonsystematic movement of the larynx [28]. In addition, CTV-to-PTV margin of 3 mm used in the current study may not be large enough to properly cover organ motion of the glottic larynx [29]. Further studies are needed to decide the exact principle for target volume delineation.
The current study has several limitations. First, the patient cohort of the current study has a short follow-up period. Although late local recurrence is possible, this early result retains some significance as the cohort has a longer median follow-up period than previously reported median times to local recurrence [3,5,15,30]. Further follow-up is warranted for evaluation of late toxicities. Second, due to the retrospective nature of the current study, toxicities may have been underreported. Identifying toxicities from the medical records was primarily based on subjective symptoms, which may affect accuracy. Bias may have been present by analyzing both prospective and retrospective cohorts, as underreporting the events could be more profound in retrospective cohort. Third, although we described the relationship of acute mucositis and the hypopharyngeal mean dose, the current study could not quantify the exact benefit of excluding posterior commissure from the CTV because hypopharyngeal dose limitation was not always defined during inverse planning process. Without proper hypopharyngeal dose limitation, even the exclusion of posterior commissure from the CTV may result high hypopharyngeal dose with minimal benefit. Finally, most patients (85.7%) included in the current study had T1 disease, which has a good prognosis. The dose fractionation scheme with high BED used in the current study would be more effective and suitable for T2 disease. Despite these limitations, the current study showed the efficacy of hypofractionated regimen and potential principle of CTV delineation.
In conclusion, hypofractionated RT with 3.5 Gy per fraction for early glottic cancer is effective, with low rate of severe toxicities. No recurrence was reported in the current study. The hypopharyngeal mean dose could predict the occurrence of grade ≥2 acute mucositis. The posterior commissure may be safe to exclude from the CTV, which may reduce the occurrence of acute mucositis. We expect to report additional data with larger cohort and longer follow-up for confirmation of the findings from the current study as well as evaluation of long-term toxicities in future.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization: HGW, JHL, SKK, EJC; Funding acquisition: HGW; Investigation and methodology: HGW, JHL, SKK, EJC; Project administration: HGW, SKK; Resources: HGW, JHL, SKK, EJC; Supervision: HGW, SKK; Writing of the original draft: HGW, THL; Writing of the review and editing: THL; Software: THL; Validation: HGW, THL; Formal analysis: THL, JHL, SKK; Data curation: THL; Visualization: THL. All the authors have proofread the final version.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2019R1F1A1040583) and the Ministry of Science and ICT (No. NRF-2020M2D9A1093990).