Bone-only oligometastatic prostate cancer: can SABR improve outcomes? A single-center experience
Article information
Abstract
Purpose
Ablative treatment of oligometastases has shown survival benefit with certain tumors, although these effects still are to be demonstrated in prostate cancer.
Materials and Methods
We analysed the toxicity and clinical control results obtained in patients with bone-only oligometastatic prostate cancer treated with stereotactic ablative radiotherapy (SABR). Retrospective study on patients with metachronous oligoprogression and synchronous de novo bone-only oligometastatic prostate cancer treated with SABR and androgen deprivation therapy.
Results
Treatment schedules varied according to location and organs at risk, with biologically equivalent dose (BED) ≥100 Gy. Fifty-five bone lesions (31 patients) were treated and evaluated for toxicity, local control, progression-free survival (PFS), and overall survival (OS). After a 41-month follow-up, there was minimal acute or chronic toxicity and no G3 toxicity. The local control at 3 and 5 years was 100% and 87.1%, respectively. Median PFS and OS were 43 and 98 months, respectively. The best result in PFS was obtained with BED ≥230 Gy, delaying time to the next systemic therapy by 28.5 months.
Conclusion
The use of SABR in bone oligometastases of prostate cancer is safe with minimal toxicity and excellent results in local control and PFS, delaying the start of the next systemic therapy.
Introduction
Worldwide, prostate cancer is the second most common cancer in men and the fifth leading cause of cancer-related deaths [1]. Historically, the treatment for metastatic prostate cancer has been androgen deprivation therapy (ADT) followed by systemic treatment when this failed. In addition, the use of docetaxel was a major milestone in increasing the overall survival (OS) and clinical response rate in metastatic castration-resistant prostate cancer (mCRPC) [2]. Subsequently, the advent of androgen receptor-targeted agents (ARTAs) has brought about a major breakthrough in this setting [3–6] as well as in metastatic hormone-sensitive prostate cancer (mHSPC) [7–10].
However, not all metastatic patients progress the same way. There is a subgroup with a limited number of lesions with less aggressive behaviour corresponding to the oligometastatic state, defined by Hellman and Weischselbaum [11] as an intermediate state between a localised tumour and widespread metastatic disease.
This patient profile may produce better results when non-invasive and potentially immunogenic local treatment of metastases with stereotactic ablative radiotherapy (SABR) is added to their therapeutic regimens [12]. Furthermore, metastasis-directed therapy (MDT) with SABR in these patients is well tolerated with minimal toxicity, delaying clinical progression with a potential benefit in both metastasis-free survival and new systemic therapy, as demonstrated in several retrospective studies and randomised phase II trials, such as ORIOLE [13] and STOMP [14]. Increasing the time to new systemic therapy could increase quality of life in these patients [15,16].
In the case of prostate cancer, metastasis-targeted therapy has been evaluated in mHSPC and mCRPC, with good tolerance and promising results [17,18], although most studies include patients with nodal involvement and few focus on exclusive bone metastases, despite having a cancer-specific mortality 1.58 times higher than exclusive nodal metastases and being the sole site of metastasis in 63.6% of metastatic patients [19]. Next-generation imaging (NGI) tests detect metastases with lower prostatic specific antigen (PSA) levels, allowing earlier diagnosis of these patients [20,21].
The aim of this study was to assess the clinical control and toxicity of SABR on exclusive bone metastases PCa, as well as the time to the next-line systemic treatment (NEST).
Materials and Methods
We retrospectively evaluated patients with ≤5 exclusively bone metastases from prostate cancer, treated at our centre with SABR in the context of oligometastatic disease. All the patients were evaluated by a multidisciplinary committee and signed informed consent for the treatment and for the anonymised use of their data for scientific purposes. Eight patients debuted with de novo oligometastatic disease ("synchronous group") and the remaining 23 presented with metachronous disease ("metachronous group"). In the first group, seven patients were treated with ADT, external beam radiation therapy (EBRT) for the primary tumour (doses of 76 Gy/38 fractions, 70 Gy/28 fractions, or 62 Gy/20 fractions) and SABR for metastases, while the remaining one received ADT and SABR for metastases. In the other group, 15 patients were treated at diagnosis with EBRT ± ADT and eight underwent surgery ± EBRT ± ADT. The presence of metachronous bone metastases was diagnosed after confirming biochemical recurrence, following the Phoenix criteria [22] in cases treated with EBRT, and the European Association of Urology criteria [23], in surgical patients. After confirming biochemical progression, the imaging tests available at the centre were performed in each case, confirming the presence of metastases: computed tomography (CT) or whole-body magnetic resonance (WBMR) and bone scintigraphy and choline-PET-CT. All patients received ADT for 6 months (metachronous) or 24 months (synchronous) concurrent with irradiation.
Prior to ablative treatment, a planning CT scan was performed using 3-mm thick slices. Each patient was treated with customised immobilization devices and in some cases a 4D-CT scan was performed. The CT was co-registered with the available diagnostic tests for each patient. The planning target volume (PTV) was obtained by adding a 5-mm margin to the bony lesion in all directions. For vertebral lesions, the recommendations published by the International Spine Radiosurgery Consortium [24] were followed. Dose to PTV was prescribed at 100% (complying D98% ≥100% and D2 ≤110% of the prescribed dose). Treatment was delivered by intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT), flattening filter free to reduce delivery times on linear accelerator. A kilovoltage cone-beam CT (kV-CBCT) was performed on all the patients prior to treatment to verify the positioning. Once the patient is positioned, a slow CBCT is performed, and the clinician manually adjusts the target for accuracy. We stratified the patients by the biologically equivalent doses (BED) received in the SABR treatment: the median BED (α/β: 1.5) was 230 Gy.
Weekly clinical check-ups were carried out during the treatment and 15 days after its conclusion. After 3 months, a further follow-up with PSA and the same imaging test used at diagnosis was performed to assess the patient's response. Thereafter, follow-ups were programmed every 6 months with an associated imaging test if progression was suspected. Acute and chronic toxicity was determined according to the Common Terminological Criteria for Adverse Events (CTCAE) version 5 [25].
The statistical analyses were carried out with SPSS software (v24.0; IBM Corp., Armonk, NY, USA) and the following factors were evaluated: local progression-free survival (LPFS) understood as relapses within the irradiation field (defined as an increase in the size or uptake of the treated lesions detected in the PET-CT together with an increase in the PSA values), PFS (defined as an increase in PSA >25% post-SABR or the appearance of a new lesion on imaging tests), OS (defined as the time elapsed from the last day of SABR treatment to death from any cause or the last follow-up), and time to the NEST (defined as the time from the end of concurrent ADT treatment with SABR to progression and the start of the subsequent systemic treatment).
Survival analyses were estimated by applying the Kaplan-Meier method, performing the log-rank test to assess differences between the different categories analysed. Cox regression was performed to assess variables that could impact survival. p-values ≤0.05 were considered statistically significant.
Results
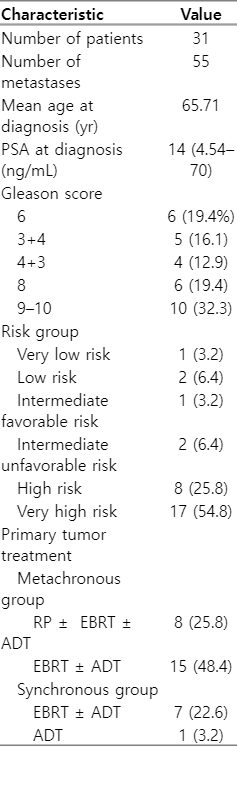
At the time of diagnosis, the mean age was 65.7 years (range, 45 to 77 years); 80.6% of patients were high or very high-risk, and the median PSA was 14 ng/mL (range, 4.5 to 70 ng/mL). The median time to progression for metachronous lesions was 71 months (range, 14 to 143 months). The median PSA at the time of progression (pPSA) prior to the procedure was 3.7 ng/mL (range, 0.3–97.0 ng/mL). Fourteen lesions were synchronous, 41 were metachronous, and 81.8% were non-spinal. A total of 55 bone lesions were treated in 31 patients (18 patients were treated with SABR on a single bone lesion, 5 patients on two bone lesions, 5 patients on three lesions, and 3 patients on four bone metastases). The baseline patient characteristics are shown in Table 1.
The most frequent location of metastases was in the iliac bone (34.5%), followed by ribs (16.4%) and pubic bone (10.9%). The location of the lesions is shown in Table 2. The dose and number of fractions administered varied depending on the location and restrictions of the organs at risk. The dose range received (BED1.5) was 130–275 Gy and the most used treatment schedule was 30 Gy/3 fractions (SABR characteristics are shown in Table 2). The treatment was well tolerated and there were no cases of ≥G3 toxicity; 43.6% of the cases presented acute G1 toxicity and 7.3% experienced G2 toxicity (mainly urinary) due to the treatment of the primary tumour. There was no chronic toxicity in 63.6% of the cases and there was only one G2 case.
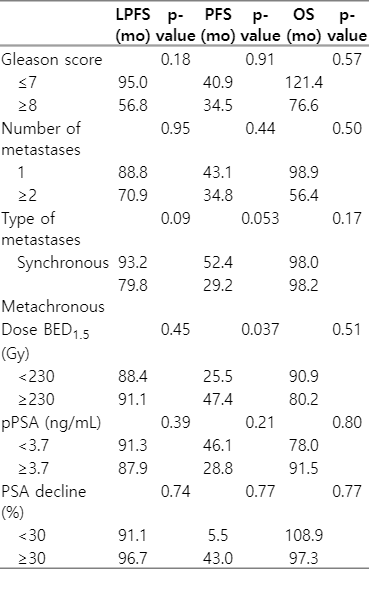
The median follow-up time was 41 months (range, 1 to 160 months). The LPFS rate was 94.5% and relapse was only observed in three lesions. Local control at 3 and 5 years was 100% and 87.1%, respectively. BED ≥230 Gy had no impact on local control in the multivariate analysis (hazard ratio [HR] = 2.38; 95% confidence interval [CI], 0.21–26.33; p = 0.47), probably due to the small number of events in our series. The other variables analysed showed no trend in the univariate or multivariate analysis, probably due to the limited number of events.
The median PFS was 43 months (95% CI, 19.4–66.5), with 35.2% of patients currently free of disease. Furthermore, the PFS was higher when the BED was stratified (47.4 months vs. 25.5 months in BED ≥230 Gy vs. <230 Gy, respectively; p = 0.037). A trend in PFS was observed in synchronous versus metachronous lesions (52.4 months vs. 29.2 months; p = 0.053), while no differences were found according to the pPSA levels (p = 0.21), number of metastatic lesions (p = 0.44), Gleason index (p = 0.91), or risk group (p = 0.11). Moreover, BED remained a significant factor for PFS in the multivariate analysis (HR = 2.92; 95% CI, 1.18–8.41; p = 0.046) (Fig. 1).
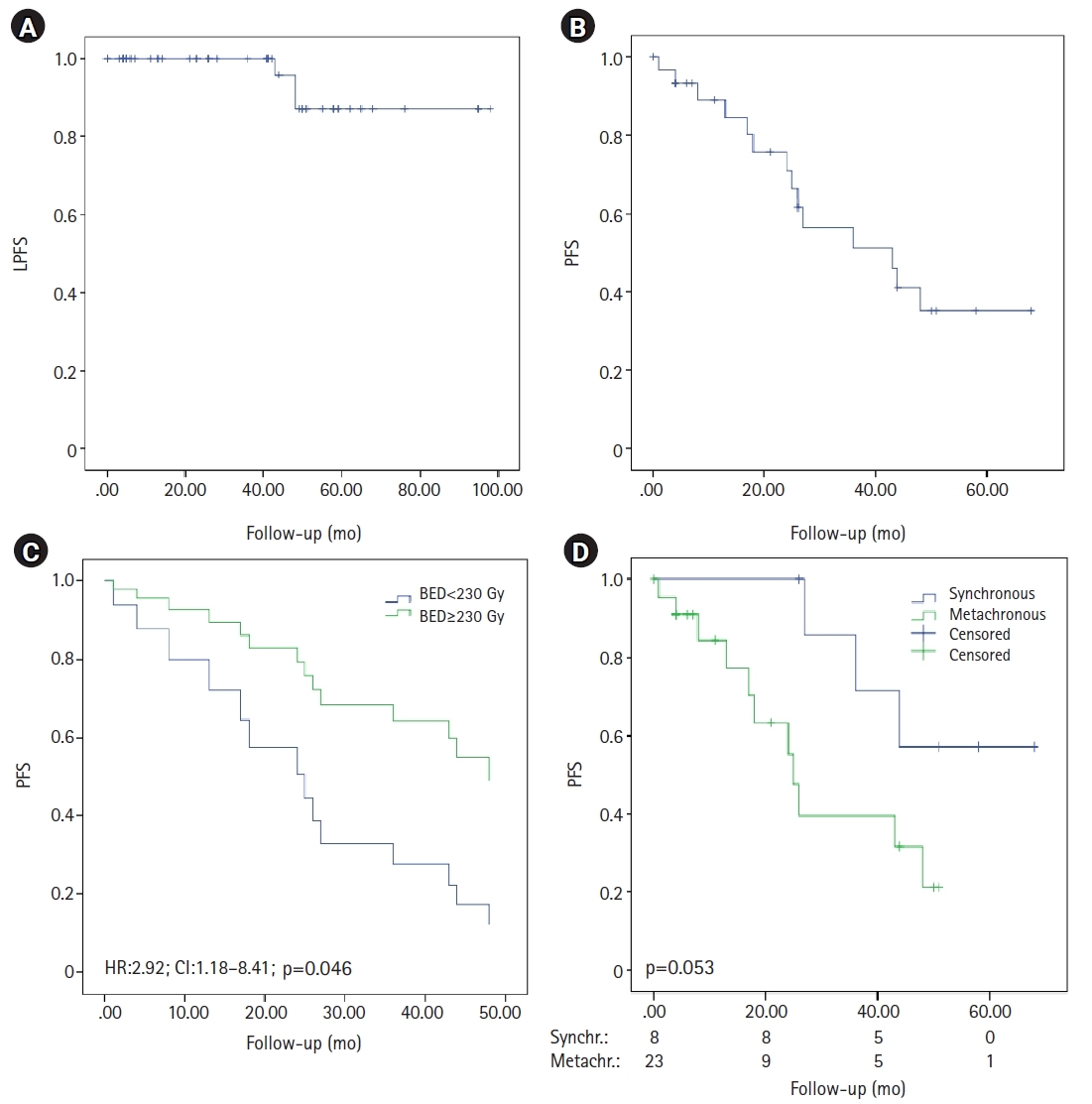
Kaplan-Meier curves of survival. (A) Local progression-free survival (LPFS). (B) Progression-free survival (PFS) and (C) Cox regression in patients with BED <230 Gy and ≥230 Gy. (D) PFS in patients with synchronous oligometastatic disease and metachronous oligorecurrent disease. BED, biologically equivalent dose; HR, hazard ratio; CI, confidence interval.
The most frequent progression location was distant (30.9%), followed by lymph nodes and mixed locations (5.5% each). The treatments received were ARTA (22.58%), SABR (12.9%) and ADT (12.9%). Metastasis-free survival (MFS) was 61.8%, with a median of 48 months (95% CI, 32.3–63.6). At the time of analysis, 77.4% of the patients were still alive, with a median OS of 98 months (95% CI, 18.1–177.8). There were four cancer-specific deaths and the OS at 3 and 5 years was 82.6% and 77.4%, respectively. We did not find any significant differences in the OS when we considered the decrease in PSA after SABR (p = 0.77) (prognostic factors for LPFS, PFS and OS are shown in Table 3).
Finally, in our results we observed a delay in the initiation of the NEST, at 28.5 months.
Discussion and Conclusion
In some solid tumour types, local SABR treatment of oligometastatic patients has recently shown survival benefit in a phase II trial (SABR-COMET) [26]. In oligometastatic prostate cancer, the data we have so far show that MDT is safe for the patient, with a good tolerability profile and delaying clinical progression [13,14]. In this study, we assessed metastasis-targeted therapy with SABR and ADT in patients with bone-only oligometastatic prostate cancer, with satisfactory results in terms of toxicity, local control and time to the NEST.
This study has some limitations, the first being its retrospective nature with a small patient cohort compared to other studies of metastasis-directed therapy, since it was a single-center study, with a small number of patients fulfilling the condition of exclusively bone oligometastatic disease. Other limitations may be heterogeneity in terms of the imaging techniques used (the large majority of choline-PET and a small part conventional imaging tests) and the presence of a minority of synchronous lesions compared to metachronous lesions. On the other hand, a highlight of our study is that it only includes exclusive bone lesions compared to the higher frequency of both bone and lymph node lesions studies in the literature.
Our work on oligometastatic bone disease exclusively treated with SABR shows that patients had excellent tolerance to the treatment with only low rates of acute or chronic G2 toxicity, and no cases of ≥G3 toxicity. There was only one case of acute G2 toxicity related to the treatment of the metastases, in the form of pain. These tolerance data correspond with those reported elsewhere in the literature [27,28].
Ablative treatment of bone metastases offers excellent local control, as shown by Habl et al. [29] and Rogowski et al. [30], with the latter reporting a local failure rate of 1.7% at 24 months. Our data are consistent with these previous findings, with an LPFS of 100% at 36 months. Of particular note, our analysis shows that the number of metastases, Gleason index, stage at diagnosis, or pPSA levels were not statistically significant predictive factors for survival. The PFS was higher when we stratified the patients according to the BED, which should therefore be considered an independent predictor of PFS after multivariate analysis. BED >100 Gy have been considered as ablative doses in different tumour primaries. Ost et al. [31] found a correlation between BED exceeding 100 Gy and PFS in cases of prostate oligometastases, and Hurmuz et al. [32] reported the same correlation in LPFS. Our data confirmed the correlation with PFS, but not with LPFS. There was a trend towards higher PFS in patients with synchronous lesions (p = 0.053); no differences were found in BED received (p = 0.78) or in lesions per patient (p = 0.60), finding a lower age (p = 0.019) and a lower percentage of toxic habits in the subgroup of synchronous patients.
All our patients received ADT during the ablative treatment, but we do not know its possible impact on the results. Some authors [29] did not observe differences if hormonal treatment was added, although Rogowski et al. [30] observed a benefit in patients who underwent ADT during SABR. This provides a possible form of study that would let us compare our series with another group receiving exclusively SABR.
Radiation therapy to the primary tumour improved the OS in patients with metastatic prostate cancer with a low tumour burden, as seen in the HORRAD trial [33] and STAMPEDE trial [34]. Furthermore, a secondary analysis [35] of the STAMPEDE trial data revealed that the number of bone metastases influenced the OS, with this metric being more relevant in patients with ≤3 lesions. Therefore, in patients with bone oligometastases, adding ablative treatment of metastases to radiotherapy of the primary tumour, we would likely achieve greater local control of the disease, which could lead to a benefit in terms of OS. Nonetheless, although the data we obtained in our series showed a trend (p = 0.172) in this subgroup of patients, we still lack randomized studies to support this assumption.
Time to next systemic therapy is being used as an exploratory outcome in metastases directed therapies for different tumour types. In two studies of MDT in oligometastatic non-small cell lung cancer, SABR was reported to delay the time to the next systemic therapy [15,16]. Other studies have shown the synergistic action of sequential MDT and ADT in prolonging the castration-sensitivity time and delaying the time to NEST [36,37]. In our series, the time to the next systemic therapy time was 28.5 months and therefore represented promising data that may impact on the quality of life of these patients, who are elderly.
Low PSA levels after SABR on metastases have been associated with a better prognosis [38]. In the setting of mCRPC, an early decrease in PSA levels 4 weeks after the onset of abiraterone acetate may function as a surrogate for OS and PFS [39,40]. However, in our work, we assessed the early decrease in PSA after SABR and did not obtain significant differences in PFS or OS in patients with a decrease in PSA by ≥30% at 1 month compared to lower decreases.
In conclusion, our data allow us to conclude that ablative treatment of bone metastases in patients with oligometastatic prostate cancer is a safe treatment that produces minimal toxicity and excellent results in terms of local control. Higher BEDs were associated with better PFS results, thereby delaying the start of the next systemic treatment.
Notes
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Castellón Provincial Hospital (Protocol Code: CEIC-108-03).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Financial Support
This study was funded by the Castellón Provincial Hospital Foundation.
Author Contributions
Conceptualization, ASI, VMM. Methodology, VMM, ASI. Investigation, VMM, ASI, ASJ. Writing—original draft preparation, ASI, VMM. Writing—review and editing, ASI, VMM, CFA. Funding acquisition, ASI. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data are available on request from the corresponding author.