Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor
Article information
Abstract
Purpose
Past studies have reported that S-allylcysteine (SAC) inhibits the migration and invasion of cancer cells through the restoration of E-cadherin, the reduction of matrix metalloproteinase (MMP) and Slug protein expression, and inhibition of the production of reactive oxygen species (ROS). Furthermore, evidence is emerging that shows that ROS induced by radiation could increase Met activation. Following on these reports of SAC and Met, we investigated whether SAC could suppress Met activation.
Materials and Methods
Wound healing, invasion, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium (MTT), soft agar colony forming, western blotting, and gelatin zymography assays were performed in the human nasopharyngeal cancer cell lines HNE1 and HONE1 treated with SAC (0, 10, 20, or 40 mM) and hepatocyte growth factor (HGF).
Results
This study showed that SAC could suppress the migration and invasion of HNE1 and HONE1 cell lines by inhibiting p-Met. An increase of migration and invasion induced by HGF and its decrease in a dose dependent manner by SAC in wound healing and invasion assays was observed. The reduction of p-Met by SAC was positively correlated with p-focal adhesion kinase (p-FAK) and p-extracellular related kinase (p-ERK in both cell lines). SAC reduced Slug, MMP2, and MMP9 involved in migration and invasion with the inhibition of Met-FAK signaling.
Conclusion
These results suggest that SAC inhibited not only Met activation but also the downstream FAK, Slug, and MMP expression. Finally, SAC may be a potent anticancer compound for nasopharyngeal cancer treated with radiotherapy.
Introduction
Nasopharyngeal cancer (NPC) is a malignant tumor that originates from the nasopharyngeal epithelium and has a high incidence in Southern China and Southeast Asia. During the early stages of NPC there are few symptoms, and therefore patients are usually diagnosed at the advanced stages. The standard treatment of advanced NPC is cisplatin based concurrent chemoradiotherapy (CCRT), where the 5-year overall survival (OS) was 72.3% in a major clinical study [1]. Regarding the expression of Met which is a proto-oncogene product, a receptor tyrosine kinase, and a specific receptor for hepatocyte growth factor (HGF), 5-year OS was 48.0% in the high-expression group while it was 84.0% in the low-expression group [2]. This was because the expression of the Met receptor and HGF were increased found to be increased in advanced stage NPC, and the c-Met/HGF pathway may stimulate proliferation, invasion, and metastasis [3]. In addition, overexpression and activity of the Met oncogene by ataxia telangiectasia mutated, the nuclear factor kappa B signaling pathway, and stromal cell-like fibroblasts could be induced by ionizing radiation (IR) [4]. A recent study also demonstrated that irradiation induced Met overexpression and activation in NPC cells [5]. From previous studies we identified two factors relevant to CCRT of NPC patients having Met overexpression that should be considered for future therapy. They were to reduce the overwhelming reactive oxygen species (ROS) by IR and to suppress the c-Met/HGF pathway.
S-allylcysteine (SAC), a derivative of garlic, has low toxicity and stable oral bioavailability and acts as an antioxidant through various mechanisms [6]. In addition, anticancer effects of SAC were reported in prostate cancer, breast cancer, oral cavity cancer, non-small cell lung cancer, neuroblastoma, and hepatocellular carcinoma through the restoration of E-cadherin, the reduction of Slug and matrix metalloproteinase (MMP) protein expression, and stimulating apoptotic pathways [789101112]. From these studies, it might be proposed that SAC reinforces the positive effects, whilst counteracting the negative effects of CCRT. The major anticancer effects of SAC were to suppress cancer proliferation, invasion, and metastasis but their mechanisms in relation to the Met signaling pathway were not investigated.
In this study, we analyzed whether SAC could suppress the proliferation, invasion, and migration stimulated by HGF in the NPC cell lines HONE1 and HNE1.
Materials and Methods
1. Cell culture and reagents
The human nasopharyngeal cell lines HONE1 and HNE1 were provided by Dr. Ronald Glaser (The Ohio State University Wexner Medical Center, Columbus, OH, USA). Human keratinocytes (HaCaT cell line) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). HONE1 cell lines were maintained in Roswell Park Memorial Institute 1640 medium (Gibco-Invitrogen, Carlsbad, CA, USA), and the HNE1 and HaCaT cell lines in Dulbecco's Modified Eagle's Medium (DMEM). The medium was supplemented with 10% fetal bovine serum (FBS; Gibco-Invitrogen). The cell lines were incubated in an incubator at 37℃ and 5% CO2. SAC was purchased from BOC Sciences (Shirley, NY, USA) in Korea. Phosphate-buffered saline (PBS) was used as a solvent control. Recombinant human HGF was purchased from R&D System Inc. (Minneapolis, MN, USA). Collagen type I was purchased from Gibco-Bethesda Research Laboratories Inc. (Carlsbad, CA, USA).
2. Wound-healing assay
Cells were plated on 24-well plates and grown to confluency, followed by serum deprivation for 24 hours. Wounds were generated by using a sterile 1,000-µL pipette tip and washed with 1 × PBS. Cells were then treated with SAC (0, 10, 20, or 40 mM) and HGF for an additional 24 hours. Three wells per experimental treatment were examined at ×10 magnification by light microscopy. Each assay was performed in triplicate.
3. Invasion assay
Cell invasiveness was evaluated using collagen-coated transwell membrane inserts (8.0 µm pore polycarbonate membrane). The transwells were obtained from Corning Inc. (Corning, NY, USA). HONE1 or HNE1 cells were plated on the insert. Both inserts and lower wells were treated with vehicle control (PBS), SAC (0, 10, 20, or 40 mM), and HGF. After 24 hours, the stationary cells were removed from the top side of the membrane, whereas the migrated cells on the lower side of the membrane were fixed with methanol and stained with hematoxylin and eosin. The number of invading cells was counted in four representative fields per membrane by using light microscopy at ×40 magnification. Each assay was performed in triplicate.
4. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium (MTT) assay
The cells were seeded at a density of 3,000 cells per well in 96-well plates containing 100 µL of growth medium and different concentrations (from 1 to 40 mM) of SAC or PBS (as the control). For each analysis eight replicates were used and at least three independent experiments were performed.
5. Soft agar colony formation assay
HNE1 cells were diluted to 5 × 103 cells/mL in 0.4% noble agar solution in DMEM with 20% FBS. The cell suspension was added to each well of a 24-well plate on an underlayer of 0.8% noble agar in DMEM with 20% FBS at 37℃. Fresh DMEM containing 0.2% FBS with or without HGF (10 ng/mL) was added to the top of the soft agar and then cultured at 37℃ for 21 days. The culture medium was changed twice per week with or without HGF. The colonies were stained with a 0.05% crystal violet solution (dissolved in 20% methanol) and the stained colonies were counted using the MetaMorph NX image software (Molecular Devices, Sunnyvale, CA, USA). Each experiment was performed in triplicate.
6. Western blot analysis
Cell lysates were prepared and western blot analysis was performed as described previously [13]. Western blot analysis was performed using the following primary antibodies: p-Met (pY1234/1235), Met, extracellular related kinase (ERK), p-ERK, focal adhesion kinase (FAK), p-FAK, Src, and Slug (Cell Signaling Technology, Beverly, MA, USA). All antibodies were used at a 1:1000 dilution.
7. Gelatin zymography
MMP2 and MMP9 were assayed by gelatinolytic activity by means of gelatin zymography. The conditioned medium was mixed with sodium dodecyl sulfate (SDS) sample buffer-50 mM Tris HCl (pH 6.8), 10% glycerol, 1% SDS, 0.01% bromophenol blue-in the absence of a reducing agent to denature MMPs. The mixture was then incubated at 37℃ for 20 minutes, and SDS-polyacrylamide gel electrophoresis, containing gelatin at a final concentration of 0.1%, was performed. After electrophoresis, the gel was washed twice with 2.5% Triton X-100 for 30 minutes at room temperature and then incubated for 24 hours at 37℃ in a developing buffer containing 50 mM Tris HCl (pH 7.6), 150 mM NaCl, 10 mM CaCl2, and 0.02% NaN3. The MMPs were identified following staining of the gel in 0.125% Coomassie Blue R-250 (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 40% methanol, 10% acetic acid and destaining in the same solution. Gelatinolytic activity was visualized as a clear band against a dark background of stained gelatin. MMP2 was detected by the clear band appearing at 72 kDa and MMP9 at 92 kDa.
8. Statistical analysis
Statistical evaluation of the data was conducted using Student t-test. Results were considered as statistically significant at p < 0.05.
Results
1. SAC inhibits the migration and invasion of NPC cells in co-treatment with HGF
To examine the inhibitory effects of SAC on the migration and invasion ability of HNE1 and HONE1 cells induced by HGF, we performed a wound healing assay (Fig. 1A) and a collagen-coated transwell invasion assay (Fig. 1B). As shown in Fig. 1A, in the wound healing assay we scratched the growing HNE1 and HONE1 cells in 24 well plates and then pretreated with SAC for 24 hours, followed by HGF (10 ng/mL) treatment for 24 hours. HNE1 and HONE1 cells were each grouped by SAC concentration to 4 groups (0, 10, 20, and 40 mM). HGF induced narrowing of the scratch wound in both cells. The percentage of closed area increased from 39.1% to 72.8% in the HNE1 (1.86 fold) and from 63.3% to 100% in the HONE1 cell line (1.58 fold). However, co-treatment of SAC with HGF resulted in less wound closure than after treatment with HGF alone and the percentage of closed area decreased as the concentration increased. The closed area decreased from 72.8% to 43.8% in the HNE1 and from 100% to 90.8% in the HONE1 cell line treated with both HGF and 10 mM SAC. Along the same lines, we evaluated the invasion of HNE1 and HONE1 cells in a transwell invasion assay. HGF induced invasion in both cells. The relative increase of invasion cell numbers ± standard error (SE) was 2,150% ± 750% in the HNE1 and 67.6% ± 28.2% in the HONE1 cell lines. Co-treatment of SAC with HGF significantly inhibited cell invasion compared with HGF alone. The relative reduction of invasion cell numbers ± SE was 66.7% ± 11.3% in the HNE1 and 36.7% ± 5.8% in the HONE1 cell lines treated with both HGF and 10 mM SAC. This meant that SAC inhibited HGF induced cell migration and invasion.
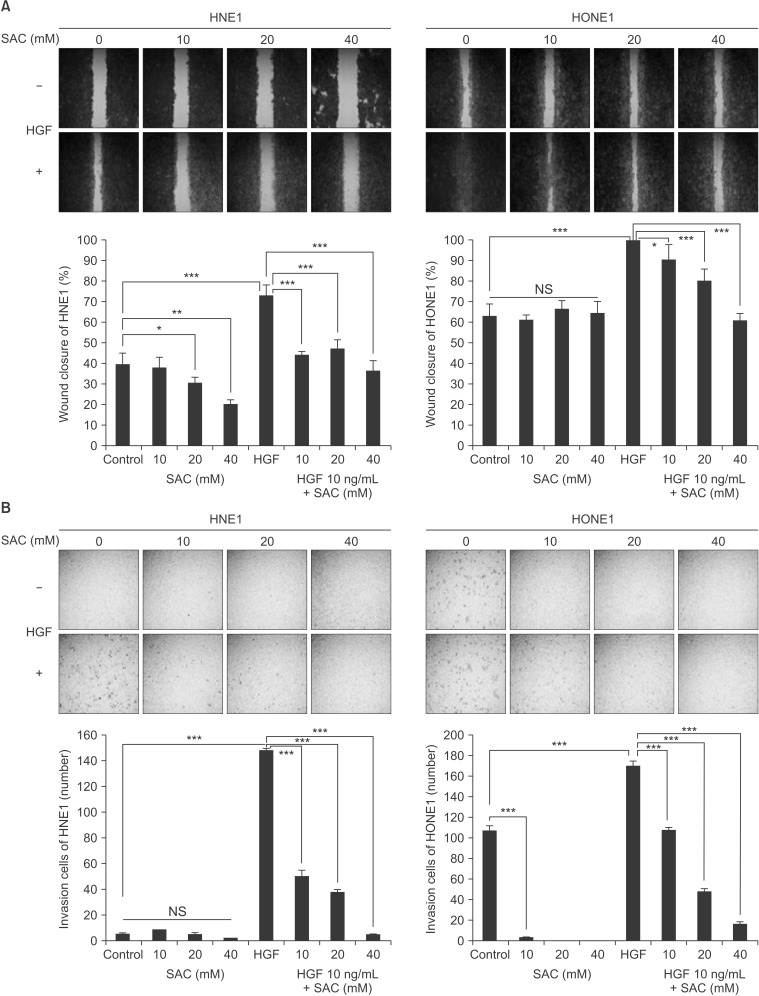
S-allylcysteine (SAC) inhibited dose-dependently the wound healing and invasion induced by hepatocyte growth factor (HGF) in HNE1 and HONE1 cell lines. (A) Wounds of confluent cells seeded in 24-well plates were generated using a sterile 1,000-µL pipette tip in HNE1 and HONE1 cell lines. The cells were then washed with phosphate buffered saline and treated with HGF (10 ng/mL) alone, SAC (10, 20, or 40 mM) and HGF, or SAC alone (10, 20, or 40 mM) for 24 hours. (B) Cells were seeded in a collagen-coated transwell plate and incubated with 1% fetal bovine serum medium with HGF (10 ng/mL) alone, SAC (10, 20, or 40 mM) and HGF, or SAC alone (10, 20, 40 mM) for 24 hours. After 24 hours the invading cells were stained and counted. Data shown represent the mean ± standard deviation. NS, not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
2. SAC reduces anchorage dependent and independent growth induced by HGF in NPC cells
We conducted MTT assays to find the most effective dose and duration of treatment to inhibit the growth of HNE1 and HONE1 cells (Fig. 2A). As dose was escalated and time was prolonged, cell numbers decreased. The growth inhibition of SAC in HONE1 cells showed significant difference between 24, 48, and 72 hours (p < 0.05) from SAC concentrations of 10 mM to 40 mM, while that in HNE1 cells did not. Median lethal concentration of HNE1 and HONE1 cells at 24 hours was about 10 mM. We conducted a soft agar colony forming assay with or without HGF to verify the inhibitory effect of SAC on the anchorage independent cell growth in HNE1. As shown in Fig. 2B, HNE1 cells stimulated by HGF grew well in soft agar, but co treatment with SAC and HGF in HNE1 significantly decreased colony size and number. This result supports the assertion that SAC suppressed cancer cell transformation.
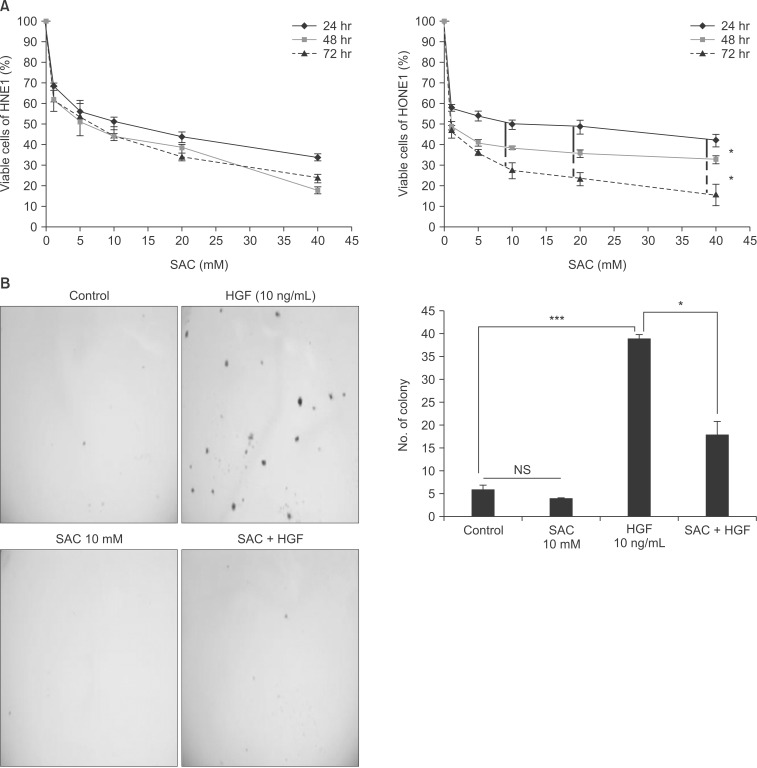
S-allylcysteine (SAC) suppressed dose-dependently the proliferation of HNE1 and HONE1 cell lines. SAC inhibited colony forming ability induced by hepatocyte growth factor in HNE1 cell line. (A) MTT assay was performed at 24, 48, and 72 hours in HNE1 and HONE1 cells. (B) Soft agar assay in which cells were seeded at a density of 2.5 × 103 cells/mL and cultured in 0.4% fetal bovine serum at 37℃ for 21 days in HNE1. SAC was added to the soft agar every 3 days. After 3 weeks, the colonies were stained with 0.05% crystal violet. The stained colonies were counted using MetaMorph NX image software. Data shown represent the mean ± standard deviation. MTT, 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium; NS, not significant. *p < 0.05, ***p < 0.001.
3. SAC suppresses FAK by inhibiting the phosphorylation of Met in NPC cells
We performed western blotting after co treatment of SAC and HGF to verify the inhibitory effect of SAC on Met/HGF signal transduction in HNE1 and HONE1 cells. SAC dose-dependently reduced the Met tyrosine phosphorylation on pY1234/1235 (p-Met) stimulated by HGF. Furthermore, we also analyzed the downstream signaling of Met, FAK, Src kinase, and ERK activation. As shown in Fig. 3A, p-Met decreased dose dependently in both cells stimulated by HGF. The FAK phosphorylation on pY397 (p-FAK) was reduced by SAC in HNE1 cells with or without HGF at 2 hours after treatment. The Src phosphorylation on pY416 (p-Src) was decreased by SAC with or without HGF in HONE1 cells, and the reduction of p-FAK by SAC was observed without HGF but not with HGF 2 hours after treatment in HONE1 cells. There was no increase of p-FAK with HGF alone and there was a reduction of p-FAK 2 hours after HONE1 cells were treated with HGF and 40 mM SAC. A clear decrease of p-FAK, total FAK, and p-Src by SAC, with or without HGF, was observed after 24 hours in HONE1 but not in HNE1 as shown in Fig. 3B. An increase of p-Src was present in HONE1 cells treated with HGF alone after 2 hours but not at 24 hours, while p-FAK increased with HGF alone at 24 hours. Slug protein was also reduced at 24 hours with or without HGF treatment in the HONE1 cell line, while it was reduced only in the 40 mM SAC treatment, with or without HGF, groups. There was no significant increase with HGF only HNE1 cells as shown in Fig. 3B. Active MMP2 and MMP9 were decreased by SAC in both cells treated with HGF and the difference was clearer in HNE1 than in HONE1 cells (Fig. 3C).

S-allylcysteine (SAC) inhibited the activation of Met and downstream signaling by hepatocyte growth factor (HGF). (A) HONE1 or HNE1 cells were pretreated with 0, 10, 20, or 40 mM SAC for 2 hours followed by treatment with 10 ng/mL HGF for 10 minutes. The cells were then harvested and western blot analysis was performed for p-Met, Met, p-extracellular related kinase (p-ERK), ERK, p-focal adhesion kinase (p-FAK), FAK, p-Src, and Src (shock protein 60 was used as a loading control). (B) HONE1 or HNE1 cells were treated with HGF (10 ng/mL) alone, SAC (10, 20, or 40 mM) and HGF, or SAC alone (10, 20, or 40 mM) for 24 hours. The cells were then harvested and western blot analysis was performed for Slug, p-FAK, FAK, p-Src, and Src. (C) HONE1 or HNE1 cells were treated with HGF (10 ng/mL) alone, SAC (10, 20, or 40 mM) and HGF, or SAC alone (10, 20, or 40 mM) for 24 hours. The cells were then harvested and matrix metalloproteinase-2 (MMP2) and MMP9 activity was assessed by gelatin zymography.
Discussion and Conclusion
Met is one of the prognostic factors for advanced NPC [14]. This is because the proliferation, invasion, and migration of cancer cells is increased through the Met/HGF signaling pathway [15]. There have been many studies that aimed to develop compounds targeting the Met receptor and they demonstrated that the proliferation, migration, and invasion of cancer could be suppressed by inhibiting the stimulation of Met receptor [16]. However, IR, a major modality of nasopharyngeal caner, causes problems like normal tissue damage and activation of cell growth signals including the Met signaling pathway by excessive ROS [145]. Therefore, it would be ideal to solve both problems of Met and ROS. Previous studies supported that SAC had both an antioxidant and an anticancer effect at a similar dosage range in mM [1718]. In the present study we found that SAC suppresses the proliferation, invasion, and migration in NPC cell lines. Further investigation showed that this is related to the down regulation of p-FAK and p-ERK by inhibiting p-Met.
The proliferation of NPC cells was reduced anchorage dependently and independently by SAC. This effect could influence wound healing and invasion assays. However, SAC's ability to inhibit migration and invasion was not as significant as its suppression of proliferation, as assayed by MTT, when HGF was not administered (Fig. 1 and Fig. 2A). Namely, antiproliferation was not correlated with suppression of migration and invasion by SAC in both cell lines. The decrease of anchorage independent proliferation was more effective in cells with HGF than those without, but this reduction was not enough to reach cell death (Fig. 2B). This might be related to the inactivation of Met but not the major active pathway of SAC in this study. The p-Met was increased by HGF and its decrease by SAC occurred along with the reduction of p-ERK 2 hours after treatment in both cells (Fig. 3A). At that time, p-FAK was increased by HGF and decreased by SAC, but the p-Src was not correlated with p-FAK in HNE1. There was no association between p-Src and p-FAK in HNE1 cells 24 hours after treatment (Fig. 3B). On the other hand, p-FAK was not changed, while p-Src was increased by HGF. In addition, p-Src was decreased by the increase of SAC in HONE1 without a clear reduction of p-FAK (Fig. 3A). The total FAK and p-FAK were influenced by p-Src at 24 hours in HONE1 cells stimulated by HGF (Fig. 3B). The increase of p-Src stimulated by HGF 2 hours might be connected to the increase of p-FAK at 24 hours in the HONE1 cell line. These results meant that p-FAK was directly affected by p-Met in HNE1, and p-FAK was indirectly affected by p-Src mediating p-Met in HONE1. A previous study demonstrated that direct interaction of FAK with Met was required for FAK to promote HGF induced cell invasion in lung cancer cells [19]. That indirect interaction between Met, Src, and FAK leads to cell migration in mammary cell carcinoma has been well established [15]. Furthermore, past studies showed that receptor tyrosine kinase-FAK signaling was mediated by Slug or Snail to disrupt the adherence junction [20]. In addition, the association between Slug and MMP2 or MMP9 is well known in multiple cancers [2122]. From these studies we concluded that Slug and MMP2 or MMP9 are downstream to Met-FAK signaling. Slug was decreased only by 40 mM SAC in HNE1, but by all concentrations in HONE1 at 24 hours (Fig. 2B). Wound healing and invasion due to HGF were more prominent in HNE1 and it was also inhibited to a greater extent in HNE1 than in HONE1 cell (Fig. 1). In addition, the increase of MMP2 and MMP9 by HGF and its decrease by SAC was clearer in HNE1 than in HONE1 cells (Fig. 2C). The difference between these phenotypes in terms of migration and invasion might be due to differences in the timing of SAC effects on p-FAK, Slug, and MMPs; namely, this result might be because the development of the Met-FAK signaling pathway was more delayed in HONE1 than in HNE1 cells. The delay might occur whether p-FAK was mediated by p-Src or not. A previous study in the same cell lines using a Met antibody showed that the decrease in Slug started after 6 hours in HNE1 and 12 hours in HONE1, and this supports the idea of delayed effects of SAC in HONE cells [23]. We inferred that the migration and invasion of NPC cells was suppressed through the Met-FAK signaling pathway as illustrated in Fig. 4.
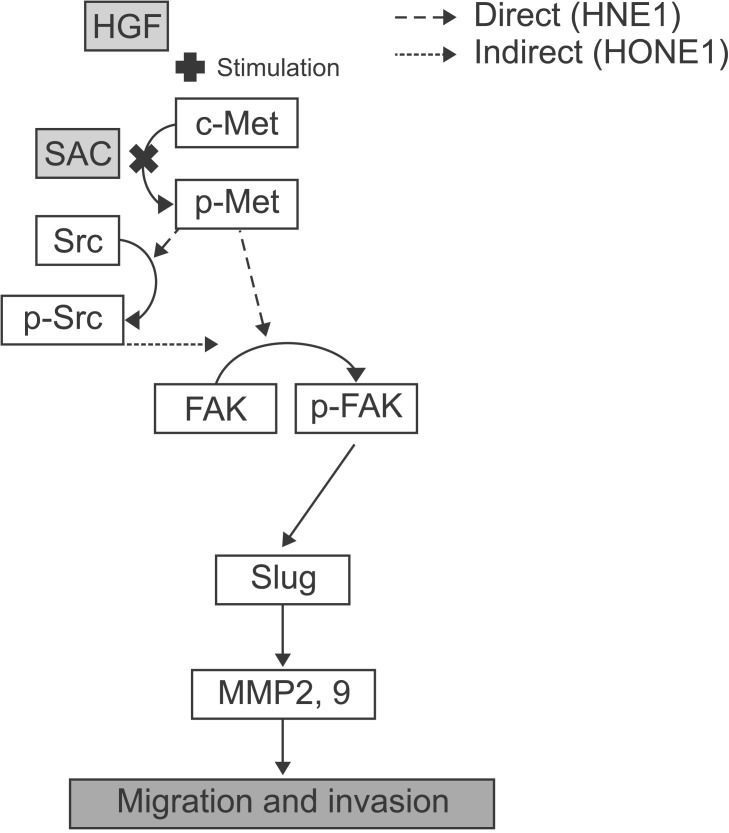
S-allylcysteine (SAC) inhibited phosphorylation of Met in the Met-focal adhesion kinase (FAK) signaling pathway stimulated by hepatocyte growth factor. HNE1 showed direct phosphorylation between Met and FAK while HONE1 presented indirect phosphorylation via Src. HGF, hepatocyte growth factor; MMP, matrix metalloproteinase.
Ferraro et al. [24] suggested that pro-metastatic signaling triggered by c-Met and mediated by ROS is linked to metastasis in melanoma. In addition, Jagadeeswaran et al. [25] reported that direct treatment of hydrogen peroxide induced the phosphorylation of c-Met in small cell lung cancer. These studies supported the association between the Met pathway and ROS and SAC, therefore, might be related to the mechanisms of ROS in the phosphorylation of Met. Therefore, additional studies on the association between ROS and p-Met would be required to elucidate the mechanisms of SAC.
It is well known that radiation induced cell death is mediated by ROS and that SAC might protect cancer by suppression of ROS. We tested whether SAC had such a protective effect in a cell viability assay of HaCaT, an immortalized human keratinocyte cell line, HNE1, and HONE1 depending on radiation and it seemed to have an enhancing effect rather than a protective effect (Fig. 5). We inferred that SAC might have no protective effect in rapidly proliferating cells. The association between SAC and ROS induced by radiation needs to be evaluated by further research.

Cell viability (%) of HaCaT (A), HNE1 (B), and HONE1 (C) cells in S-allylcysteine (SAC; 0, 1, 5, 10, or 20 mM) according to radiation (0 or 8 Gy) was plotted. The cells were treated with SAC within 1 hour before irradiation and harvested 24 hours after irradiation. Radiation was delivered using 6 MV photon of Varian 21EX. Data shown represent the mean ± standard deviation. ***p < 0.001.
This study had three limitations. First, an in vivo study was not included. Further investigation into the effect of SAC in vivo is certainly required. Second, the mechanism by which SAC affects p-Met was not provided in this study. Further detailed investigations in vitro are required for identifying this mechanism. Third, there was no study about whether SAC could scavenge ROS induced by radiation and affect Met-FAK signaling pathway in irradiated NPC cells instead of HGF despite many studies confirming its antioxidant effects [6]. Despite these imitations, this paper suggests a new finding: that SAC suppresses migration and invasion by inhibiting the Met-FAK signaling pathway in NPC cells. This finding was significantly related to CCRT, the standard treatment of NPC, because SAC is an antioxidant and both Met and ROS might be associated with radioresistance induced by IR [45].
In conclusion, SAC can control the migration and invasion induced by HGF in NPC cell lines. This is achieved by the effective degradation of Met. This could have clinical relevance for NPC treated with CCRT. Therefore, SAC may be a potent anticancer compound for advanced or recurrent nasopharyngeal cancer.
Acknowledgments
This work was supported by 2012 grant from "the Department of Medical Sciences at the Graduate School of the Ajou University" and the Bio & Medical Technology Development Program of the NRF funded by the Korean government (2012M3A9B2052870).
Notes
The result of this study was presented at the regular congress of the Korean Society for Radiation Oncology (KOSRO) held in Seoul, Korea on October 16-17, 2014.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.