Radiomics in stereotactic body radiotherapy for non-small cell lung cancer: a systematic review and radiomic quality score study
Article information
Abstract
Purpose
Stereotactic body radiotherapy (SBRT) has been widely utilized for curative treatment of early-stage non-small cell lung cancer (NSCLC). It has achieved good local control rate comparable to surgery. Currently, no standard risk model exists for SBRT outcome or complication prediction. Radiomics has the potential to improve clinical outcome prognostication. Here, we reviewed the current literature on the radiomic analyses of thoracic SBRT through the use of radiomic quality score (RQS).
Materials and Methods
Literature search was conducted on PubMed and Embase to retrieve radiomics studies on SBRT for early NSCLC. The literature search included studies up to June 2021. Only full papers published in peer reviewed journals were included. Studies that included metastatic lung cancers or non-lung cancers were excluded. Two independent investigators evaluated each study using the RQS and resolved discrepancies through discussion.
Results
A total number of 25 studies were analysed. The mean RQS was 7.76 of a maximum score of 36. This corresponds to 21.56% of the maximum score. Lack of feature reduction strategies, external validation and open data sharing were identified as key limitations of the reviewed studies. Meanwhile, various common radiomic signatures across different studies such as gray level co-occurrence matrix Homogeneity and energy have been identified. Multiple robust radiomic models have also been reviewed that may improve outcome or complication prediction.
Conclusion
Radiomics in thoracic SBRT has a very promising future as a prognostication tool. However, larger multicenter prospective studies are required to confirm radiomic signatures. Improvement in future study methodologies can also facilitate its wider application.
Introduction
Stereotactic body radiotherapy (SBRT) has been applied in the treatment of early-stage non-small cell lung cancer (NSCLC). It has also been used in the treatment of solitary thoracic metastases [1]. Clinical trials have demonstrated that good local control of over 90% can be achieved for early-stage lung cancer [2]. This is comparable to outcomes achieved from surgery [3].
Distant failure is the most common mode of failure with a significant impact on overall survival [4]. Various studies have attempted to elucidate risk factors for failure. Size has been shown to be an important prognostic factor in which tumors less than 20 mm were shown to have favorable prognosis [5]. Squamous cell carcinoma has been shown to convey a poorer prognosis compared to adenocarcinoma [6]. Pre-SBRT fluorodeoxyglucose standardized uptake value (SUV) value is also predictive of distant failure with SUV cut off beyond 5 indicating increased risk for distant failure [7]. In terms of local failure, radiation dose and fractionation were important. A 54–60 Gy over 3 fractions was associated with superior local control compared with alternative fractionation schemes [5]. Other prognostic factors include pre-SBRT hemoglobin level; sex and performance status [5,8]. Patients with adverse risk factors can be followed up more intensively for both local and distant failures.
Despite the growing body of research in SBRT prognostication, it is crucial to have more sophisticated models in order to select patients who can benefit the most from SBRT. This would also allow high-risk patients to undergo more intensive surveillance.
1. Radiomics
Imaging is now an integral part of cancer management. In fact, images such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) provide vast amounts of important clinical information. Radiomics represents a significant advancement in precision medicine. The central hypothesis of radiomics is that imaging features can be useful in providing prognostic information. Computer algorithms can now extract shape, statistical, and textural information from radiographic images. Through the analysis of radiomic features, a thorough analysis of the tumor can be done in order to construct clinically useful models.
Radiomics allows more subtle features that are not appreciated by the naked eye, to be characterized. Such information allows sophisticated models to be built, which will vastly improve diagnostic and prognostic accuracy.
It has been shown that radiomic features can be used to predict overall survival (OS) and distant metastasis (DM) in various malignancies [9,10]. It has also been studied in SBRT outcome prediction in pancreatic cancer, hepatocellular carcinoma, and pulmonary oligo-metastases [11-13]. Correlations between histology, gene mutation, and radiomic features have been found in various studies [14,15]. Recently, treatment response towards immunotherapy has been predicted with good accuracy using radiomic features [16]. Likewise, studies have demonstrated a relationship between treatment complications and radiomic features such as in the case of radiation pneumonitis (RP) [17-19]. Overall, radiomics has improved prognostication and outcome prediction in a wide range of malignancies.
2. Workflow
Radiomic analysis can be applied to all sorts of images including CT, PET, MRI, and ultrasound. After acquiring images, clinicians are required to delineate the region of interest (ROI) for further analysis. The ROIs are not limited to primary tumors; radiomic analyses have also been done on peritumoral ROIs and organs at risk.
After determining the ROI, radiomic features can be extracted from the ROIs. The features can be broadly divided into three categories: shape-based, statistical-based, and textural-based features.
3. Statistical features
Statistical features refer to the statistical description of voxel intensities within the ROI. As such, a basic statistical overview of the ROI can be obtained. Common features include mean, maximum, minimum, standard deviation, range, skewness, and kurtosis. Subsequently, the statistical distribution of the voxel value can be mapped out.
4. Shape textures
Shape textures refer to the description of the geometry of the ROI. Such features can be calculated in 2D, and it can also be rendered in 3D. Common features include volume, surface area, diameter, sphericity, elongation, and flatness.
5. Textural features
Textural features also referred to as second-order features, are concerned with the interrelationship of surrounding voxels. Different matrices can be constructed in order to measure the statistical relationship of surrounding voxels. For example, the gray level co-occurrence matrix (GLCM) measures the incidence of co-occurrence of voxels of equal intensity over a given distance. Gray level run length matrix measures the number of consecutive voxels with equal intensity over a given distance. Other matrices include gray zone size zone matrix, gray level dependence matrix and neighbouring gray tone difference matrix. All these matrices quantify the texture of the ROI to determine the heterogeneity of the ROI. Ultimately, the extracted radiomic features can be used to construct models for prediction or classification.
Materials and Methods
In the present study, we reviewed the current body of literature on the radiomic analyses of SBRT in early NSCLC. A literature search was performed for studies on the application of radiomic analysis in SBRT of early NSCLC. A PubMed and Embase search was performed, in which all papers up to June 2021 were included. The keywords were “SBRT” OR “SABR” AND “RT” OR “NSCLC” OR “Lung” AND “Radiomic.” Search results were reviewed, in which only radiomic studies on the use of SBRT in lung cancers were included. Studies including metastatic lung cancers or non-lung cancers were excluded. Studies involving systemic therapy were also excluded. Only the papers that were published in peer-reviewed journals in English were included in this analysis. Non-original investigations such as reviews were excluded from the analysis. The reference lists of each included study were screened manually for other suitable studies.
The included studies were further stratified according to their goal. Study quality was evaluated by the radiomic quality score (RQS) proposed by Lambin et al. [20]. RQS evaluates the quality of study through a series of 16 objective criteria. Possible scores range from -8 to 36, as scores can be deducted if feature reductions or model validation are not done. The items of the RQS were presented in Supplementary Table S1. Two independent readers blinded to the studies then scored the RQS for each study. After the initial scoring, the readers discussed each study. Whenever there was a scoring discrepancy, the readers reached a consensus through discussion.
Results
One hundred twenty-seven studies were identified in our literature review. Twenty-five studies were included after exclusion. Some of these studies analyzed outcome prediction, while other studies analyzed complication prediction. Of the 25 studies, 21 studies used radiomics to predict SBRT clinical outcomes. Four studies predicted complications arising from SBRT.
Median number of subjects was 87 (range, 13 to 573). The median number of extracted radiomic features was 44 (range, 1 to 1,605). The mean RQS was 7.76 (range -3 to 16; confidence interval, 5.53–9.98). This RQS corresponds to 21.56% of the maximum score. This scoring was in line with other RQS studies on other primary sites [21-23].
All studies provided well-documented imaging protocols (25/25, 100%). However, none of the studies utilized public protocols. Nine studies (9/25, 36%) performed multiple segmentation during ROI delineation. Three studies (3/25, 12%) analyzed imaging at multiple time points. None of the studies accounted for inter-scanner variability through a phantom. A significant number of studies did not perform feature reduction (9/25, 36%). Less than half of the studies (11/25, 44%) included non-radiomic features in their analyses. None of the studies provided a biological correlation of radiomic features. All studies (25/25, 100%) supplied discrimination statistics for its classification or prediction. However, only a minority of studies (5/25, 20%) provided cutoff analysis. Model validation is an important aspect of radiomics studies. However, a significant number of studies (6/25, 24%) did not perform any validation on its models. Two studies (8%) validated the results through external data from distinct institutes. One study (4%) validated results using data from multiple institutes. Other studies relied on internal validation. None of the studies compared its models with the gold standard. No cost-effectiveness analyses were done for all studies. Finally, no data or code in the reviewed studies were publicly available.
Discussion and Conclusion
1. Radiomic for SBRT outcome prediction
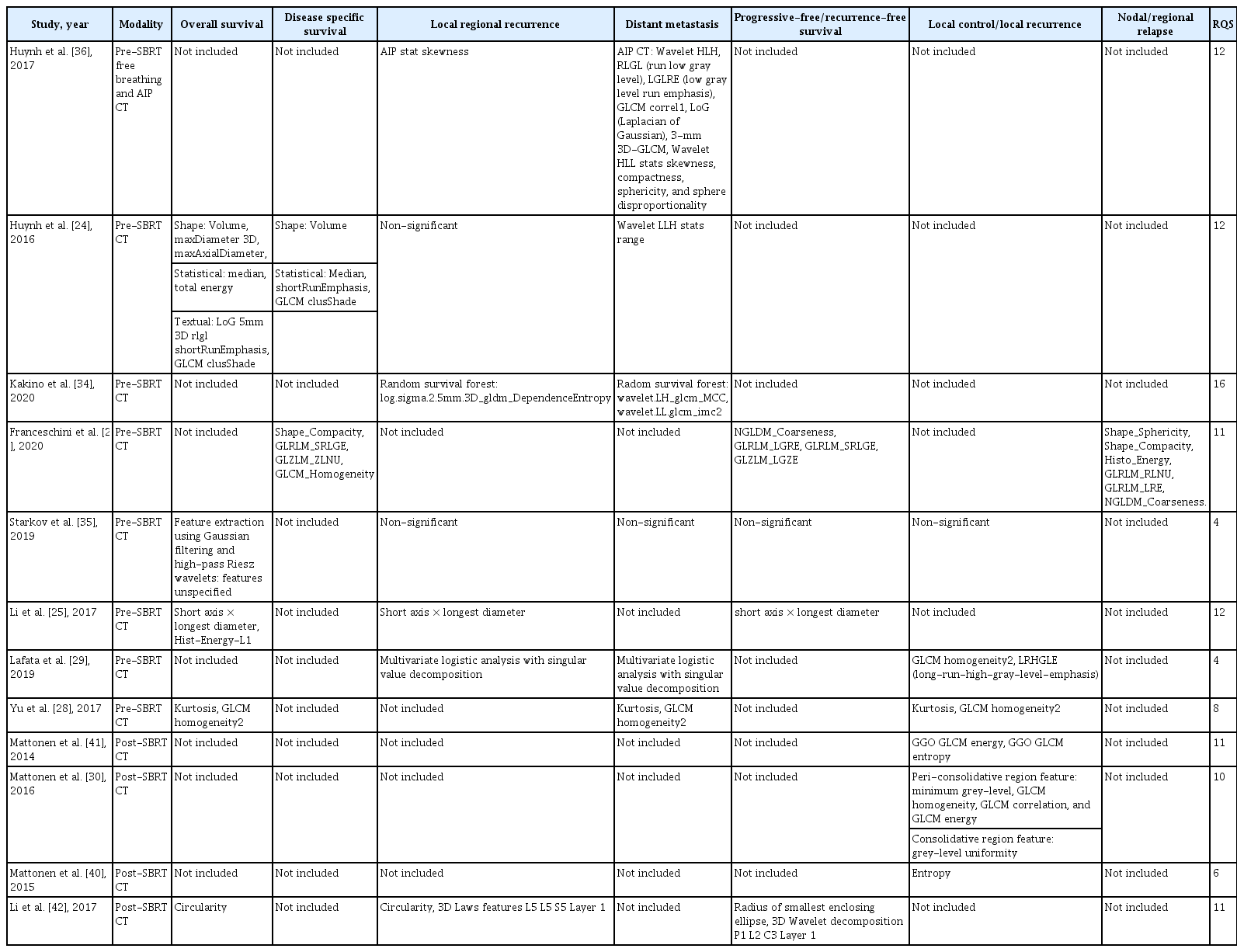
Twenty-one studies on radiomic-based SBRT response prediction were reviewed here. The imaging modalities used in the studies included CT, PET-CT, and MRI. Twelve studies evaluated CT-based radiomics, eight studies were on PET-CT-based radiomics while one study was on MRI-based radiomics. A wide range of outcomes were investigated including overall survival (OS), disease-specific survival (DSS), recurrence-free survival/progression-free survival (RFS/PFS), local control (LC), regional control (RC), local regional control/recurrence (LRR), and DM.
The predictive radiomic features identified in each study were not always consistent. This may be due to the use of different feature extraction protocols and feature sets in studies. The most commonly identified prognostic feature across studies was GLCM homogeneity, which was identified in four different studies. This was followed by GLCM correlation, GLCM energy, GLCM entropy, sphericity, and kurtosis. The findings in each study have been summarized in Table 1.
2. Pre-treatment CT
Most studies utilized pre-SBRT CT radiomics for outcome prediction, in particular the pre-SBRT gross tumor volumes (GTVs). This approach may be useful to select patients who may benefit the most from SBRT [24-35]. OS and DSS were important outcomes to be predicted. Huynh et al. [24] demonstrated that shape, statistical, and textual features were all associated with OS. Shape features such as volume, maxDiameter 3D, and maxAxial Diameter were predictive of OS [24]. Statistical features such as median and total energy have been identified in the same study as predictive factors. Textural features have also been identified including run low gray level (RLGL) shortRunEmphasis and GLCM clusShade. RLGL measures the distribution of low gray level within the area of interest. DSS shared similar radiomic features.
Similar prognostic radiomic features were found by a study led by Li et al. [25], in which short axis × longest diameter, Hist-Energy-L1 were identified. Short axis × longest diameter roughly corresponds to the volume feature in the study by Huynh et al. [36] as they both indicate the size of the tumor. This strongly affirms the key prognostic implication of tumor size in thoracic SBRT [26]. Meanwhile, Hist-Energy-L1 and total energy both measure the magnitude of voxel value within the ROI. Energy was also found to be prognostic in predicting nodal relapse in thoracic SBRT in a separate study [27].
Yu et al. [28] developed a radiomics model on a cohort of stage I NSCLC patients receiving surgery as curative treatment. The same model was then validated in an independent cohort of patients with stage I NSCLC receiving thoracic SBRT. Two prognostic radiomic features were validated, including kurtosis and GLCM homogeneity. In fact, these two features were both prognostic for OS, DM, and LC.
GLCM homogeneity was shown to be prognostic for OS, DSS, DM, and LC in four separate studies for stage I NSCLC treated with SBRT [27-30]. This feature measures the closeness of pixel pairs. An increase in GLCM homogeneity was found to confer a worse prognosis as homogeneity was positively correlated with mortality risk [28]. The robustness of GLCM homogeneity across studies indicates that it may be an important radiological biomarker for thoracic SBRT in early NSCLC.
However, a separate study led by Coroller et al. [31] performed a radiomic analysis of a cohort of stage II-III NSCLC patients receiving neoadjuvant chemo-irradiation followed by surgery. The study analyzed predictive radiomic features of the primary tumor for gross residual disease and pathological complete response. Involved lymph nodes were excluded from the analysis. Interestingly, GLCM homogeneity was not associated with either outcome. Although it is acknowledged that the delineation of ROI for radiotherapy in stage I NSCLC and stage II-III NSCLC may be different which may lead to the difference in result. Nonetheless, as GLCM homogeneity was only predictive in stage I NSCLC patients receiving SBRT, this may still generate the hypothesis that GLCM homogeneity is a stage-specific radiomic marker for radiotherapy. This is crucial as it was shown that stage I NSCLC patients undergoing SBRT with increased GLCM homogeneity confer worse prognosis, such patients may benefit from dose escalation or alternative therapies.
3. Post treatment CT
SBRT frequently leads to radiation-induced lung injury or pneumonitis [32]. At times, lung injury can appear like a mass-like consolidation, which is difficult to distinguish from recurrence [37]. High-risk features to differentiate recurrence include enlarging mass-like consolidation and bulging opacity [38,39]. However, such features often appear 1 year after treatment, which delays recurrence detection. Hence, another advantage of radiomics is the ability to detect local recurrence earlier.
Through a series of studies, Mattonen et al. [30,40,41] leveraged radiomics to confront this problem. Physician-driven recurrence detection was compared with radiomics-based recurrence detection [30]. Physician-based recurrence detection on post-SBRT follow-up CT had a mean sensitivity and specificity of 83.8% and 75%, respectively. However, there was suboptimal inter-observer agreement. The median time to recurrence detection for physician-driven detection was 15.5 months. On the other hand, radiomic analysis outperforms clinicians in terms of accuracy and time to detection. Minimum grey level, GLCM homogeneity, GLCM correlation, GLCM energy, and grey level uniformity were found to be useful in recurrence detection. These radiomic signatures achieved an area under the curve (AUC) of 0.85 for recurrence detection. Time to detection was also significantly improved as 76% of recurrences can be detected using post-SBRT scans within 6 months of initial treatment.
In two separate studies, Mattonen et al. [40,41] extracted radiomic features from post-SBRT ground glass opacities and consolidative areas. GLCM energy and GLCM entropy were shown to be associated with local recurrence. On top of that, a semi-automatic segmentation algorithm devised by Mattonen et al. [40] allowed computer-assisted delineation of post-SBRT consolidative and peri-consolidative areas. This was shown to be non-inferior compared to manual delineation by radiologists. Coupled with GLCM entropy as a biomarker, the system can facilitate early and accurate detection of local recurrence.
Apart from the detection of local recurrences, post-SBRT scans can provide early prognostic information. Li et al. [42] demonstrated that radiomic features of CT images as early as 3 months after SBRT are predictive of clinical outcomes including OS, RFS, and LRR. This allows earlier adaptation of treatment and follow-up strategies.
The aforementioned studies indicated that radiomic analysis of post-SBRT CT can be useful in both recurrence detection and outcome prediction.
4. PET-CT
PET-CT plays a vital role in the diagnosis and monitoring of different cancers. It is used prior to SBRT for accurate staging, as well as monitoring of disease status after treatment. Prediction of outcomes using conventional PET-CT parameters such as SUVmax yielded contradictory results [43,44].
It was postulated that the combination of PET and CT radiomics can further contribute to prognostication accuracy. Here, we review the latest radiomic studies on PET-CT radiomics. Details of the studies described have been summarized in Table 2.
Various radiomic signatures extracted from PET were prognostic. High-intensity large area emphasis (HILAE) has been shown to be associated with local control [45]. This radiomic feature may be associated with higher intra-tumoral metabolic heterogeneity.
Entropy, correlation, coarseness, busyness, and metabolic tumor volume were also among the PET parameters predictive of local recurrence [46]. Regarding survival, dissimilarity was found to be associated with both DSS and disease-free survival (DFS), while entropy was associated with DSS [46,47]. Dissimilarity refers to the variation of intensity between neighboring voxel pairs. Increased dissimilarity was found to be associated with increased RFS in another cohort of early NSCLC patients receiving either surgery or concurrent chemoradiotherapy [48].
Combined CT and PET radiomic models have also been developed. In particular, Dissaux et al. [49] combined both PET and CT radiomic features in prediction. Models combining both CT and PET radiomic features were built to predict local control. Features such as PET information correlation 2 (IC2), PET strength, and CT flatness were useful in LC prediction.
One commonly encountered problem in radiomics studies lies in the fact that myriad imaging markers are produced, resulting in redundancy of dimensionality [50]. To solve this problem, it is important to perform feature reduction, this may also improve model performance. Oikonomou et al. [51] performed principal component analysis on radiomic features extracted from both PET and CT scans to reduce feature dimensionality. This study successfully predicted OS, DSS, DFS, DM, and RC with good accuracy.
Though the dimensionality reduction technique is widely used in radiomic studies, a drawback is that some information may be lost in the process. Li et al. [52] proposed a novel concept of tumor tensor. Tumor tensor refers to the direct use of tumor images in model training instead of handcrafted radiomic features, allowing tumor information to be preserved. Ultimately, the kernel support tensor machine model outperformed traditional radiomic models in DM prediction with an AUC of 0.81.
At times, the training algorithm of machine learning models may overweigh model accuracy while sacrificing specificity. To balance the sensitivity and specificity of machine learning models, Zhou et al. [53] developed a multi-objective radiomic model in which both sensitivity and specificity were considered simultaneous during model training. A combined model of clinical, PET and CT-derived features achieved AUC of 0.83 while maintaining satisfactory sensitivity and specificity at 0.76 and 0.94, respectively. Unsupervised clustering has also been applied. Tumors with specific radiological appearance or phenotype may have multiple correlated radiomic features that naturally cluster together. Unsupervised clustering may help to unravel such a distribution. Li et al. [54] addressed this hypothesis by performing unsupervised clustering. The study identified three distinct radiomic risk groups in which the meta-features within each cluster were able to predict both OS and freedom from nodal failure.
5. MRI
Although the application of MRI in thoracic tumors is nascent, it has been widely applied in the monitoring of other malignancies such as rectal, cervical, and brain tumors. Indeed, emerging evidence suggests that MRI radiomics can play a role in the prognostication in various malignancies [55,56]. Radiological changes after SBRT often manifest in CT a few months after the treatment. MRI may be an alternate modality that can detect subtle radiological changes earlier with higher sensitivity. Sampath et al. [57] conducted a prospective trial to study the changes in apparent diffusion coefficient (ADC) before and after SBRT. Changes in ADC occurred as early as 1 month after SBRT, of which an increased ADC was associated with a higher rate of local failure. This allows local recurrence to be detected even earlier. No other MRI radiomics studies on thoracic SBRT were identified in our literature search. MRI radiomics can be a promising avenue to explore in the future.
6. Radiomics for complication prediction
Another category of studies reviewed in the current study was radiomic analysis of SBRT complications. Thoracic SBRT leads to a high rate of pneumonitis and pulmonary fibrosis [32]. As patients receiving SBRT often have more medical comorbidities, predicting RP is important. Conventional predictors of RP included lung V25, planning target volume, and mean lung dose [58,59]. Here, we review the current radiomic studies on SBRT-induced pneumonitis.
Four radiomic studies have been identified for SBRT complications. All studies were on the prediction of RP after SBRT. Different imaging modalities were used. Three studies analysed planning CT while one study analyzed post-SBRT CT. Qin et al. [60] combined both cone-beam CT during treatment and planning CT radiomics. The ROI used also differed significantly. Two studies utilized the GTV to predict RP while one study analyzed areas of increased density (consolidation or ground glass opacity). Hirose et al. [18] analysed the radiomics of total lung volume excluding the GTV. The reviewed studies were summarized in Table 3.
A wide variety of radiomic features were identified, this is likely due to the wide range of imaging modalities and ROI used. GTV first-order features such as root mean square, mean and uniformity were shown to be predictive of RP using planning CT [17]. A radiomic score comprising these features was significant in predicting RP along with age. No other clinical features were found to be associated with RP in the study. Qin et al. [60] showed that GTV shape features such as mass and orientation were predictive of RP. A significant advancement in the study was that a combined model with cone-beam CT and planning CT was built to predict RP. Subtle changes may be captured by cone-beam CT radiomics during the course of radiotherapy and such changes were shown to be prognostic of RP. This opened a new avenue for cone-beam CT-based radiomics.
Apart from GTV-based radiomics, normal lung parenchyma may have certain radiological appearances that may be predictive of radiation-induced lung injury. Following this hypothesis, Hirose et al. [18] explored normal lung as ROI instead of GTV. Radiomic features were extracted from lung volumes exposed to various levels of radiation (V0,V5,V10,V20). An ensemble averaging model was developed in which a model using radiomic features from V5 was best performing. The first-order radiomic feature correlation was found to be higher in patients with RP, indicating that it can be a radiomic marker for RP.
Apart from the first order and shape features, GLCM features including correlation, dissimilarity, and contrast were also able to distinguish the severity of lung injury [19]. A large proportion of patients receiving SBRT would develop varying degrees of RP. Although most remain asymptomatic, some may develop severe respiratory symptoms. Radiomic prediction of SBRT complication can thus facilitate patient selection prior to treatment. The literature reviewed showed a wide range of radiomic features including first order, shape, and GLCM features. The high heterogeneity may have arisen from the varied modalities and ROIs used. Further studies are required to explore optimal imaging biomarkers for RP.
7. Limitation
Radiomics is a nascent field and is far from being incorporated into daily clinical practice. Various factors limited its wider application as revealed by the current RQS analysis. The lack of validation of predictive models limits the broader application of radiomics. Nearly a quarter of the reviewed studies did not apply any form of validation. For those that validated its model, investigators often elected internal validation as the methodology. Only three studies validated its results with external data sets. The lack of external validation led to overfitting and adversely affected the generalizabiltiy of models. Larger prospective multicenter studies with dedicated validation datasets will hopefully improve the applicability of radiomic analyses.
As large amounts of radiomic features are extracted in each study, the statistical testing of the features may lead to false positive results. Ideally, 10–15 patients are required for the testing of each radiomic feature to prevent excessive false discoveries [61]. Apart from that, redundancy of features may limit the performance of multivariate models as well. In the present study, a third (9/25, 36%) of the reviewed studies did not perform feature reduction. These studies are mostly small, hypothesis-generating studies with a small number of subjects ranging from 13 to 295 subjects. The number of extracted features was small as well (1–43 features). Nonetheless, this does not eliminate the need for feature reduction, as false discoveries should be reduced as much as possible. Future studies should conduct feature reduction routinely or at least adjust for multiple testing unless the population size is sufficiently large.
Open data sharing can also improve the current state of radiomics research. None of the studies shared its data or code within a publicly available repository. This avoids external verification of study results. It also hinders confirmatory studies clarger follow-up studies may not validate its findings using previously published data.
We identified the aforementioned three factors to be the most pertinent and universal limitations as revealed in the present RQS review. Other criteria of the RQS scheme may not be applied universally to all radiomics studies as the applicability depends on the aims of the studies. Criteria such as biological correlation, cost-effectiveness analysis were not the aims of the reviewed studies. Hence, we consider these criteria to be non-essential considering the scope of the reviewed studies. There is currently no “gold standard” risk model for thoracic SBRT outcome or complication prediction. Therefore, comparison to the “gold standard” may not be appropriate assessment criteria in the current context as well.
It is also worthwhile to note that a wide range of imaging acquisition techniques and modalities are used in radiomic studies. Even within the same study, multiple machines or imaging parameters may be used. Imaging protocol for example contrast use or the phase in which images are captured may affect radiomic features as well [62-64]. There is currently a lack of guidelines standardizing imaging procedures or controlling for imaging variables in radiomic studies. This further limits the widespread clinical application of radiomics.
8. Future directions
1) Delta radiomics
The growing body of research in SBRT radiomics allows new imaging biomarkers to be discovered. Delta radiomics explores the changes in radiomic features during or after treatment as a novel class of biomarkers. For NSCLC, delta radiomics has been shown to be useful in predicting clinical outcomes in patients receiving conventional chemo-irradiation [65,66]. Similar predictions have been made in a wide range of malignancies including rectal cancer, pancreatic cancer, and soft tissue sarcoma [67-69].
Cone-beam CT is now routinely done for treatment verification in thoracic SBRT. CT thorax is also routine imaging performed after thoracic SBRT. By leveraging these routinely available images, changes in radiomic features during and after SBRT can possibly provide more valuable prognostic information.
2) Peritumoral radiomics
Peritumoral radiomics is another novel development currently explored. Radiomics of normal parenchyma surrounding tumor has been found to carry important prognostic information. This has been demonstrated in breast, lung, and liver cancers [70-72]. Recently, peritumoral radiomics has also been associated with immunotherapy response [16]. Genomic alterations associated with tumor invasion and metastasis were also found to be associated with peritumoral radiomics in lung cancer [72]. Peritumoral radiomics in thoracic SBRT may provide further prognostic value.
9. Conclusion
Radiomics represents an exciting advancement to allow non-invasive assessment of all aspects of tumor behavior. As SBRT is increasingly applied to lung cancer, radiomics may be an important assessment tool in the future. This allows more personalized treatment. More studies are eagerly awaited.
Notes
Statement of Ethics
IRB review has been waived as it does not involve subjects.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author Contributions
The author has proofread the final version.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2023.00612.
Table S1. Radiomic quality score criteria