Brain metastasis in human epidermal growth factor receptor 2-positive breast cancer: from biology to treatment
Article information
Abstract
Overexpression of human epidermal growth factor receptor 2 (HER2) is found in about 20% of breast cancer patients. With treatment using trastuzumab, an anti-HER2 monoclonal antibody, systemic control is improved. Nonetheless, the incidence of brain metastasis does not be improved, rather seems to be increased in HER2-positive breast cancer. The mainstay treatment for brain metastases is radiotherapy. According to the number of metastatic lesions and performance status of patients, radiosurgery or whole brain radiotherapy can be performed. The concurrent use of a radiosensitizer further improves intracranial control. Due to its large molecular weight, trastuzumab has a limited ability to cross the blood-brain barrier. However, small tyrosine kinase inhibitors such as lapatinib, has been noted to be a promising agent that can be used as a radiosensitizer to affect HER2-positive breast cancer. This review will outline general management of brain metastases and will focus on preclinical findings regarding the radiosensitizing effect of small molecule HER2 targeting agents.
Introduction
Overexpression of human epidermal growth factor receptor 2 (HER2) is found in about 20% of breast cancer patients and is known to have a particularly aggressive natural history. In the last decade, the introduction of trastuzumab, an anti-HER2 monoclonal antibody, has improved the survival of HER2-positive patients dramatically when used in the adjuvant setting [1]. The addition of trastuzumab to neoadjuvant chemotherapy increases pathologic complete response (CR) rate and event-free survival in locally advanced breast cancer [23]. Even in metastatic breast cancer patients, trastuzumab improves response rate and survival [4]. However, the pattern of relapse is changing, and a high proportion of patients are experiencing brain metastasis (BM) after treatment with trastuzumab.
The incidence of BM is reported to be approximately 30% in breast cancer patients. In HER2-positive patients, however, BM is diagnosed more commonly (in up to 50% of autopsy cases) [5]. In a large retrospective study of 3,726 patients with early-stage breast cancer, BM was found in 7.9%–14.3% of HER2-positive patients, compared to only in 2.2% and 4.7% of luminal A and B breast cancers, respectively. Among patients with distant disease, HER2-positive breast cancer showed a higher ratio of BM (odds ratio, 2.1–5.3) compared with luminal A breast cancer [6]. To explain this phenomenon, a potential affinity of HER2-positive breast cancer for brain tissue has been suggested. A preclinical study reported that HER2 overexpression increased brain colonization of metastatic tumor cells in vivo [7], and a pathology study using resected BM showed that the blood-brain barrier (BBB) was preserved in HER2-positive breast cancer patients [8].
After treatment with trastuzumab, the incidence of BM seems to be increased in HER2-positive breast cancer, possibly due to the fact that trastuzumab enhances systemic control and prolongs survival, and thus clinically discloses BM. According to a registry study of stage I to III breast cancer, BM was observed in 10.5% of HER2-positive patients who received trastuzumab before the diagnosis of BM, but only in 1.3% and 1.6% of HER2-negative patients and HER2-positive patients who never received trastuzumab, respectively [9]. In HER2-positive metastatic breast cancer, despite receiving trastuzumab-based therapy, approximately 30% of patients develop BM [610]. Intracranial disease progression, rather than extracranial disease, is the cause of death in about half of patients with BM [1011]. Therefore, in HER2-positive breast cancer patients with BM, control of intracranial disease is an important issue in terms of survival.
Treatments for Brain Metastasis
1. Local therapy and whole brain radiotherapy
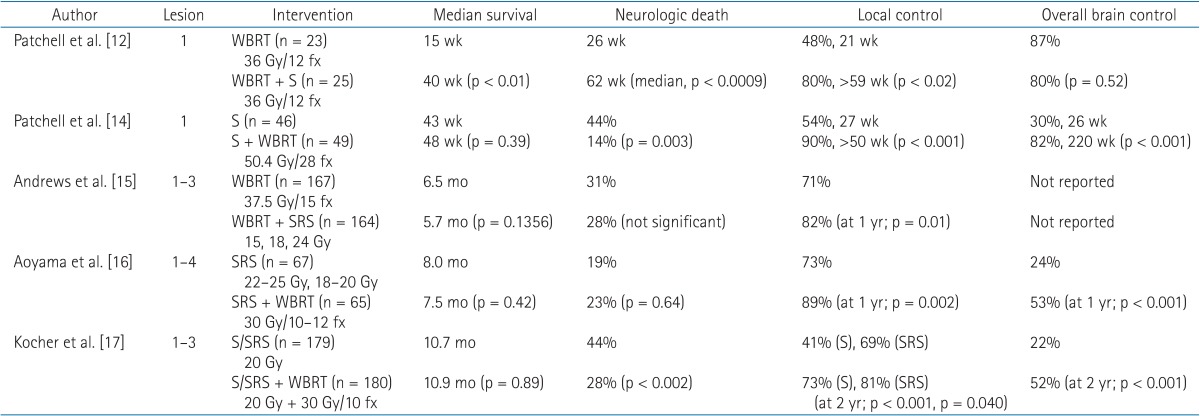
At this time, surgical excision and radiosurgery are available local treatment options for patients with 1–3 metastatic brain lesions. Surgical excision for BM can be appropriate in patients with a single intracranial lesion and controlled extracranial lesions. In these patients with good prognosis, surgical excision followed by whole brain radiotherapy (WBRT) improves survival from 15 to 40 weeks over WBRT alone [1213]. In contrast, postoperative WBRT, compared with surgery alone, decreases intracranial relapse and neurologic death but does not improve survival even in patients with a single BM [14].
Radiosurgery can be an alternative to surgery for patients with metastatic lesions at deep-seated sites or sites adjacent to critical domains. A small but significant survival benefit, from 4.9 to 6.5 months, was observed in patients with a single BM lesion with the addition of radiosurgery to WBRT [15]. For patients with two or more BM lesions, improved local control was observed for radiosurgery plus WBRT compared to WBRT or radiosurgery alone [1516]. Therefore, for patients with a limited number of BMs, the addition of WBRT to local treatment (surgical excision or radiosurgery) reduces intracranial relapses and neurologic deaths, while the survival benefit is unclear [1718]. Results of randomized clinical trials of local treatment and WBRT for 1–3 BMs are summarized in Table 1.
Randomized clinical trials of management for brain metastases: surgery (S), radiosurgery (SRS), or whole brain radiotherapy (WBRT)
For patients with four or more BMs or with uncontrolled extra-cranial disease, WBRT is the mainstay palliative treatment, and the median survival time is about 4 months [19]. Though several studies have reported the efficacy and safety of radiosurgery in patients with more than 3 BMs, none of these studies were randomized controlled, so interpretation of their findings is limited [2021]. Recently, consideration of individualized risk factors, such as overall tumor volume, performance score, and primary tumor control, rather than of traditional candidates of radiosurgery, such as the number of BMs, has been suggested in the establishment of treatment strategies [2223].
2. Whole brain radiotherapy should it be restricted?
As mentioned previously in this review, the upfront use of radiosurgery and omission of WBRT for a limited number of BMs has been suggested. This strategy is based on two concerns regarding WBRT. The first consideration is neurocognitive functional outcome after WBRT, and the second consideration is a lack of survival benefit after WBRT. As summarized in Table 1, WBRT improves intracranial control from 22%–30% to 52%–80%, as well as initial lesion control from 41%–71% to 72%–90%. More intracranial control rate equates to a decreased salvage therapy rate. In studies comparing local therapy alone vs. local therapy and WBRT, the salvage therapy rate required was 3–10 times greater in the local therapy alone group [1617]. Despite the increased use of salvage therapy, local therapy did not improve survival, but increased neurologic death. Furthermore, uncontrolled intracranial disease can influence neurocognitive functional outcomes [2425]. Aoyama et al. [26] used the Mini-Mental State Examination (MMSE) to assess neurocognitive function, and observed that the actuarial free rates of a 3-point drop in the MMSE score at 12 and 24 months were higher in patients with WBRT and radiosurgery than in patients with radiosurgery alone. The average duration until deterioration was significantly increased in patients with WBRT and radiosurgery (16.5 months vs. 7.6 months).
On the contrary, Chang et al. [27], using the Hopkins Verbal Learning Test, Revised (HVLT-R), reported that addition of WBRT to radiosurgery deteriorated short-term memory at 4 months after treatment. This result, however, should be interpreted cautiously because of insufficient patient enrollment (which was about 60% of the initial estimation) and an unexpectedly lower survival in the WBRT and radiosurgery group compared to the radiosurgery alone group (median, 6 months vs. 15 months). Health-related quality-of-life (HRQOL) results have also been compared in patients with WBRT or observation after radiosurgery [28]. Patients with radiosurgery alone have been reported to have better HRQOL than patients with additional WBRT, and these differences reached significance at the 9-month time point. However, as in the preceding report [17], patients with WBRT and radiosurgery had longer progression-free survival (PFS) and a lower salvage therapy rate, and thus these results conflict with results of the HRQOL study. Additionally, a lack of longterm data (more than 1 year) and a high drop rate (55% at 1 year) are considerable limitations. Lastly, despite the use of an exclusive neurocognitive test in this study, the impact of WBRT on neurocognitive function remains unclear.
3. Recommendations on radiotherapy for brain metastasis
Strict guidelines for managing patients with BM have not been established yet. Instead, individualized strategies should be considered depending on factors such as patient performance score, symptoms, primary disease state, systemic dissemination, number of BMs, and BM burden [29]. Particularly in patients with a limited number of BMs, the limitation of WBRT (possible neurocognitive deterioration in early phase) and the benefit of WBRT (significant enhancement of intracranial disease control and PFS) should be juxtaposed. WBRT with high precision technique can protect hippocampus without disrupting conformity, and expected to preserve memory [30]. Regardless of the technique employed, whether radiosurgery or WBRT, radiotherapy is the mainstay for management of patients with BM. In the era of HER2, an important issue is improving the outcome of radiotherapy using targeted agents as radiosensitizers.
Trastuzumab and the Blood-Brain Barrier
The BBB is a selective barrier that consists of endothelial cells, a basement membrane, and astrocyte foot processes. The permeability of the BBB decreases 100-fold as the molecular weight of the drug increases from 200 Da to 450 Da [31]. Trastuzumab has a large molecular weight (145,531 Da), thus it cannot cross the BBB. In patients with meningeal carcinomatosis, after intravenous infusion trastuzumab levels in the cerebrospinal fluid (CSF) were 300-fold lower than in serum [32]. However, the delivery of trastuzumab across the BBB might increase in certain conditions such as a disturbed BBB after radiotherapy. Animal experiments and clinical studies have reported that brain radiotherapy induces BBB permeability, and resultant changes persist from several hours to several years [3334]. Stemmler et al. [35] compared the ratios of trastuzumab in the serum and CSF before and after radiotherapy. A total of eight patients with metastatic breast cancer who presented HER2-positive were administered trastuzumab intravenously. The ratio of median trastuzumab level in the serum to CSF was 420:1 before WBRT, and it decreased to 76:1 after WBRT, which indicates that the BBB might be disturbed by radiotherapy.
Trastuzumab has a limitation to crossing the BBB, specifically its large molecular weight. A little improvement of BBB permeability might be expected by radiotherapy; however, the extent and reproducibility of such permeability changes have not been reported to be consistent. While efficacy and safety of concurrent WBRT with trastuzumab have been reported in a retrospective study (objective response rate, 74%; median survival time, 18 months) [36], a randomized trial has not been attempted. Therefore, the role of trastuzumab as a radiosensitizer during brain radiotherapy is still unclear.
Effect of Lapatinib on Brain Metastasis
1. Effect of lapatinib on brain metastasis: preclinical studies
Lapatinib ditosylate (GW572016/Tykerb; GlaxoSmithKline, Research Triangle Park, NC, USA) is a reversible dual inhibitor of the intracellular tyrosine kinase domain of HER1 and HER2. Lapatinib is expected to be used for breast cancer patients with BM because of its theoretical ability to cross the BBB resulting from its very low molecular weight (581 Da). Lapatinib is the first HER2-targeting drug that has been identified in a preclinical study to have activity against the BM of breast cancer. When epidermal growth factor receptor (EGFR)-overexpressing MDA-MB-231-BR (231-BR-HER2) brain-seeking breast cancer cells were injected in a mouse model, metastatic colonization in mouse brains was inhibited by 50%–53% in response to lapatinib [37]. Subsequently, concentrations of radioactively labeled lapatinib were validated in mice with 231-BR-HER2 brain-seeking breast cancer cells. The concentration found in the BM was 7–9 folds higher than in normal brain tissue; however, it was much lower than in peripheral metastasis (only 10%–20% according to the time from lapatinib administration) [38]. From these results, the ability of lapatinib to cross BBB and control BM has be confirmed; however, a partial restriction in its BBB permeability has also been recognized.
2. Effect of lapatinib on brain metastasis: clinical trials
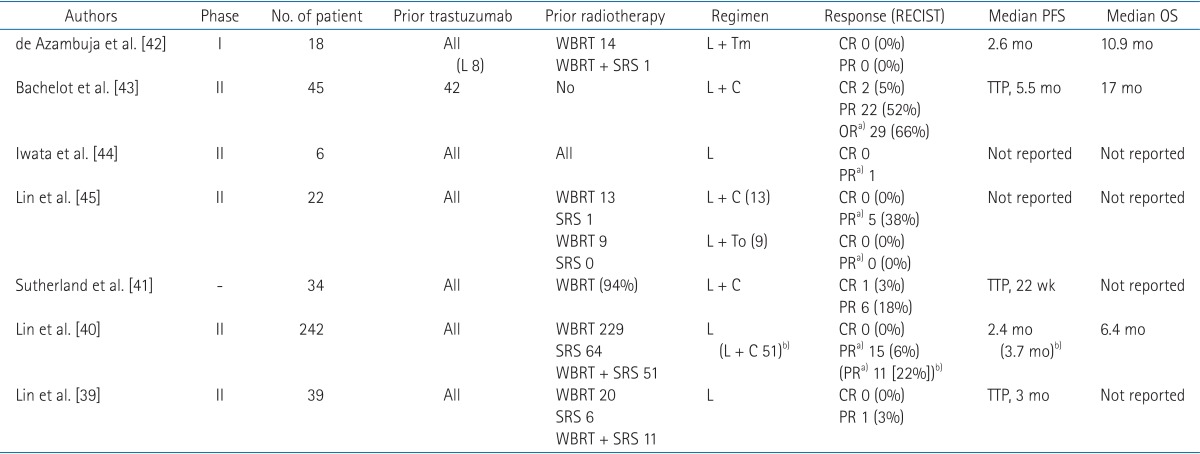
Prospective trials on lapatinib for BM in patients with HER2-positive breast cancer are summarized in Table 2 [39404142434445]. Lin et al. [39] conducted a phase II trial for patients with HER2-positive breast cancer, BM, and prior trastuzumab treatment. All patients received lapatinib treatment, and response was rated by using Response Evaluation Criteria in Solid Tumors (RECIST). The results were disappointing. No patients achieved a CR and only one patient had a partial response (PR). In a subsequent expanded study with 242 patients, 15 patients (6%) achieved an objective response (defined as ≥50% volume reduction in BMs). For patients with disease progression on single-agent lapatinib, the option of additional capecitabine was allowed. In these patients, objective response was observed in 10 of 50 patients (20%) [40]. Similarly, Sutherland et al. [41] reported a response rate of 21% in 34 patients with BM who had been administered lapatinib and capecitabine.
Results of prospective trials on lapatinib have showed a limited potential for BM patients. However, cautious interpretation is needed, since a potential delayed effect of radiation therapy may confuse the cytotoxic effect of lapatinib [46]. As shown in Table 2, almost patients in lapatinib trials underwent radiotherapy to the brain before enrollment. In contrast, Bachelot et al. [43] excluded patients previously treated with WBRT, capecitabine, or lapatinib. Among 44 patients assessable for efficacy, 29 patients (66%) achieved ≥50% volume reduction in BMs after treatment with lapatinib and capecitabine. The authors stated that the combination of lapatinib and capecitabine is an active regimen, and suggested that the combination, rather than WBRT, as an upfront strategy for treatment of BM patients with HER2-positive breast cancer. However, this trial had several limitations. First, patterns of failure in the brain were not reported. If a major component of failure is initial BMs, then an additional local modality, specifically radiotherapy, should be added. Second, the toxicity rate was high, and about half of the patients experienced grade 3 or greater adverse events. Furthermore, one of the most important sequelae, neurocognitive dysfunction, was not examined. And lastly, concentrations of drugs were not measured and compared between the serum and CSF.
Several authors have tried to validate the concentrations of lapatinib found in the CSF. Gori et al. [47] measured lapatinib concentrations in CSF and plasma of two patients with HER2-positive breast cancer treated with lapatinib and capecitabine for BM. Both patients received radiosurgery previously. The concentration ratios of CSF/plasma were significantly low, 0.9% and 1.3%, respectively. These patients did not achieve a PR and had progressive disease 14 months and 10 months after the treatment, respectively. Morikawa et al. [48] conducted a prospective study of breast cancer patients with BMs and no prior brain radiotherapy, in which they determined concentrations of capecitabine (in eight patients) and lapatinib (in four patients) in serum and resected BMs. For capecitabine, the ratio of resected BMs to serum was higher for 5-fluorouracil (5-FU) than for capecitabine, indicating that the metabolites more than the prodrugs were collected in the BMs. However, 5-FU had a wide range in terms of the ratio of resected BMs to serum, 0.28–5.64. Similarly, lapatinib had a high variability in the ratio of resected BMs to serum, 0.19–9.8. This study revealed that capecitabine and lapatinib penetrate the BBB significantly, but that the optimum level for sufficient anti-BM activity in still unclear. Also, the magnitude of permeability was highly variable among patients. Therefore, a strategy to overcome these limitations is warranted, and the authors suggested high-dose pulsatile administrations of lapatinib. Besides improving the BM uptake of lapatinib, enhancing the anti-tumor effect may be more practical by using lapatinib as a radiosensitizer.
Radiosensitizing Effect of Lapatinib
1. In vivo evidence for lapatinib contributing to the radiosensitivity of breast cancer cells overexpressing HER2
Sambade et al. [49] reported in vivo data in which mice bearing xenografts of basal-like/EGFR-positive SUM149 and HER2-positive SUM225 breast cancer cells were treated with lapatinib and fractionated radiotherapy. The treatment with lapatinib alone had no influence on tumor growth for basal-like/EGFR+ SUM149 breast cancer tumors; however, it provided significant tumor volume reduction for HER2+ SUM225 breast cancer tumors. After the combination of lapatinib plus radiotherapy (RT), mouse tumor volumes were significantly reduced in both the basal-like/EGFR+ SUM149 model and the HER2+ SUM225 model. During the study duration, treatment with both lapatinib and radiotherapy resulted in an average enhancement ratio of 1.25 for the HER2+ SUM225 model. According to immunohistochemical analyses, the radiosensitizing effect of lapatinib was associated with inhibition of AKT in the HER2+ SUM225 model.
2. Clinical evidence for lapatinib contributing to the radiosensitivity of breast cancer cells overexpressing HER2
There is only one clinical trial reporting the combination of lapatinib and WBRT, a phase I study for 35 patients with BM of HER2-positive breast cancer [50]. Most of the patients did not receive previous central nervous system (CNS) treatment: five received CNS surgery and three received radiosurgery. The primary end-point was to define the maximum tolerated dose of concurrent WBRT with lapatinib. On the first day, lapatinib 750 mg was administered twice per day. From the second day onward, three kinds of dose levels of lapatinib were administered: 1,000 mg, 1,250 mg, and 1,500 mg once daily. WBRT (37.5 Gy in 15 fractions over 3 weeks) was started in the first 8 days after the administration of lapatinib. During WBRT, patients received lapatinib continuously. After the completion of WBRT, patients were given 2 mg/kg of trastuzumab every week, combined with 1,000 mg of lapatinib every day. The trastuzumab and lapatinib protocol was continued until the progression of disease, toxicity, or withdrawal. A total of 28 patients had measurable metastatic lesions in the CNS at baseline. Among these patients, the response rate was 79% (CR in three patients and PR in 19 patients) according to RECIST criteria. With a median follow-up time of 3.8 years, the median PFS and survival times were 4.8 and 19 months, respectively. In terms of first failure sites, non-CNS failures (46%) were more common than CNS failures (23%). Toxicities of grade 3 or more, which occurred in three or more patients, were diarrhea (n = 6), nausea (n = 3), rash (n = 4), and fatigue (n = 3). On neurological assessment examined at 6 months, no significant change was observed in MMSE (12 were stable, four improved, and four become worse; p = 1.00), and neurological signs and symptoms (eight were stable, six improved, and sever become worse; p = 1.00). Quality of life was assessed at 6 months using the FACT-Br and was generally worse. Although, this study did not accomplish the maximum tolerated dose of lapatinib, because of toxicity, a high rate of CNS response was observed, indicating that lapatinib could be a good radiosensitizer in BM patients with HER2-positive breast cancer.
Ongoing Studies on the Concurrent Use of Lapatinib and Radiation
Radiation Therapy Oncology Group is performing a phase II randomized study to elucidate the effect of lapatinib with radiation on breast metastases in HER-positive breast cancer (https://clinicaltrials.gov/ct2/show/NCT01622868). Patients in the study are randomly assigned to receive WBRT with or without lapatinib. Lapatinib (1,000 mg once daily) is started on the first day of WBRT and continue throughout WBRT and 21 days after the final day of WBRT without a drug holiday. The CR rate in the brain will be assessed by a brain magnetic resonance imaging scan at 12 weeks post WBRT.
Recently, Stanford University initiated a phase II trial investigating the efficacy of lapatinib and radiotherapy in patients with locally advanced or locally recurrent breast cancer (https://clinicaltrials.gov/ct2/show/NCT01868503). Patients will receive lapatinib once daily starting 7 days before RT until completion of RT. Response rates will be assessed after the treatment.
The Hellenic Cooperative Oncology Group has designed a phase II trial to evaluate the response rate of brain metastases from lung and breast tumors under treatment with WBRT and lapatinib (https://clinicaltrials.gov/ct2/show/NCT01218529). This study is a single-arm study and in which patients will be treated with WBRT (30 Gy in 10 fractions) and lapatinib 1,250 mg once daily, followed by lapatinib 1,500 mg once daily for a total of 6 weeks.
Conclusion
With the introduction of trastuzumab, survival of HER2-positive patients has dramatically improved. However, the incidence of BMs does not be improved, rather seems to be increased in HER2-positive breast cancer. WBRT is the mainstay for BM management, and recently radiosurgery has been used in patients with limited numbers of BMs. In particular, for patients with a single BM lesion, surgical excision or radiosurgery can improve survival. The limited penetration of trastuzumab into the BBB may contribute to the increased incidence of BM in patients with HER2-positive breast cancer and CNS progression and is now emerging as a major clinical issue. Half of the patients having BM died of intracranial disease progression rather than extracranial disease following initial standard therapy with WBRT or stereotactic radiosurgery. Therefore, in these patients, if CNS control can be enhanced by more effective treatment using small molecule HER2 targeting agent(s), survival could putatively be improved.
Acknowledgements
This work supported by grants from the Korean Ministry of Health and Welfare and to Kim IA (No. 0820010).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.