Treatment outcome in patients with triple negative early stage breast cancers compared with other molecular subtypes
Article information
Abstract
Purpose
To determine whether triple negative (TN) early stage breast cancers have poorer survival rates compared with other molecular types.
Materials and Methods
Between August 2000 and July 2006, patients diagnosed with stage I, II early stage breast cancers, in whom all three markers (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor [HER]-2) were available and treated with modified radical mastectomy or breast conserving surgery followed by radiotherapy, were retrospectively reviewed.
Results
Of 446 patients, 94 (21.1%) were classified as TN, 57 (12.8%) as HER-2 type, and 295 (66.1%) as luminal. TN was more frequently associated with young patients younger than 35 years old (p = 0.002), higher histologic grade (p < 0.0001), and nuclear (p < 0.0001). The median follow-up period was 78 months (range, 4 to 130 months). There were 9 local relapses (2.0%), 15 nodal (3.4%), 40 distant metastases (9.0%), and 33 deaths (7.4%) for all patients. The rates of 5-year OS, DFS, LFS, and DMFS for all patients were 95.5%, 89.9%, 95.4%, and 91.7%, respectively. There were no significant differences in OS, DFS, LFS, and DMFS between triple negative and other subtypes (p > 0.05).
Conclusion
We found that patients with TN early stage breast cancers had no difference in survival rates compared with other molecular subtypes. Prospective study in homogeneous treatment group will need for a prognosis of TN early stage breast cancer.
Introduction
Triple-negative (TN) breast cancers are defined as tumors that do not express estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER-2). Recent gene expression profiling of breast cancer has classified breast cancer into five distinct molecular subtypes and identified clinical, biologic, and therapeutic implications based on ER, PR, and HER-2 analysis [1]; luminal A and B (ER/PR receptor positive), basal-like (negative for all three markers), HER-2 overexpressing (ER and PR negative and HER-2 positive), and normal breast-like tumor. Since a majority of basal-like cancers are TN breast cancers and approximately 80% of TN breast cancers are also basal-like breast cancers [2,3], it has been adopted that the TN and basal-like phenotypes are synonymous out of convenience [4].
TN breast cancer is known for associating with young patients and high grade tumors [5,6]. This molecular subtype also poorly responds to endocrine therapy and often grows rapidly [7]. Limited studies demonstrated that it was associated with early locoregional recurrence and increased distant metastasis [5,8-10]. However, treatment results of TN early stage breast cancer were not concordant in previous studies [5,6,11,12]. Some studies demonstrated increased locoregional recurrence in patients with TN early stage breast cancer [5,12]. Conversely, others indicated that locoregional and distant recurrence of TN early stage breast cancer after breast conserving surgery (BCS) were not different from other breast molecular subtype [6,11]. We, therefore, sought to compare the clinical outcomes in patients with TN early stage breast cancer treated with BCS or mastectomy with other types of breast cancers and to determine whether TN early stage breast cancers have poorer survival rates compared with other molecular subtypes.
Materials and Methods
Between August 2000 and August 2006, 686 patients were histologically diagnosed with invasive breast cancer at the CHA Bundang Medical Center. Of 686 patients, finally 446 patients with pathologic stage I and II breast cancers according to American Joint Commission on Cacner (AJCC) 6th ed. and with ER, PR, and HER-2 data, were retrospectively reviewed in the current analysis. All patients were treated with BCS followed by radiation therapy or mastectomy followed by radiation therapy when patients were diagnosed with pathologic T3, positive or close margin, or clinical T3 or N2 disease before neoadjuvant chemotherapy. Radiation was administered to the breast and/or regional lymphatics as clinically indicated. A prescribed dose was 50.4 Gy in 1.8 Gy fractions to the whole breast and plus a tumor-bed boost to 59.4 Gy. Of 292 patients treated with modified radical mastectomy (MRM), 15 received postmastectomy radiotherapy. Systemic therapy was administered in accordance with standard clinical practice. The chemotherapy regimens included cyclophosphamide, methotrexate, 5-fluorouracil (CMF) or anthracycline based chemotherapy including cyclophosphamide, anthracycline, 5-fluorouracil (CAF), or anthracycline, cyclophosphamide (AC) (Table 1). No patients received adjuvant trastuzumab therapy.
Patients were classified as TN if they were negative for all three receptors, HER-2 type if positive only for HER-2, and Luminal if ER or PR positive. The data on ER, PR, and HER-2 were obtained through standard clinical testing using immunohistochemistry for ER (Neomarker, Kalamazoo, MI, USA) and PR (Neomarker) and HER-2 (Neomarker). For ER and PR, receptor positivity was based on more than 10% of cells testing positive, in accordance with our standard guidelines [13]. HER-2 data were obtained through routine clinical testing. Scores of 0 and 1 were considered negative, and a score of 3 was considered positive. There is uncertainty about appropriate classification of HER-2 2+ patients. Recently, it has become common to test HER-2 2+ patients by fluorescent in situ hybridization (FISH) to determine the more appropriate classification. In the current study, we did not have FISH information. Nine patients with ER and PR negative and HER-2 2+ were excluded in this analysis.
The end points of this study were overall survival (OS), disease-free survival (DFS), locoregional-free survival (LFS), and distant metastasis free survival (DMFS). OS was defined as the time from the pathologic diagnosis date to death from any cause, DFS from the pathologic diagnosis date to relapse or death, LFS from the pathologic diagnosis to the time of first evidence of locoregional relapse, and DMFS from the pathologic diagnosis to the time of first evidence of distant metastasis. The Kaplan-Meier methods were used to calculate OS, DFS, LFS, and DMFS and univariate analysis was performed using the log-rank test. A forward stepwise Cox proportional hazards regression analysis was used for multivariate analysis. Differences between categorical variables were calculated using standard χ2 methods. A p-value of less than 0.05 was considered statistically significant.
Results
1. Patients characteristics
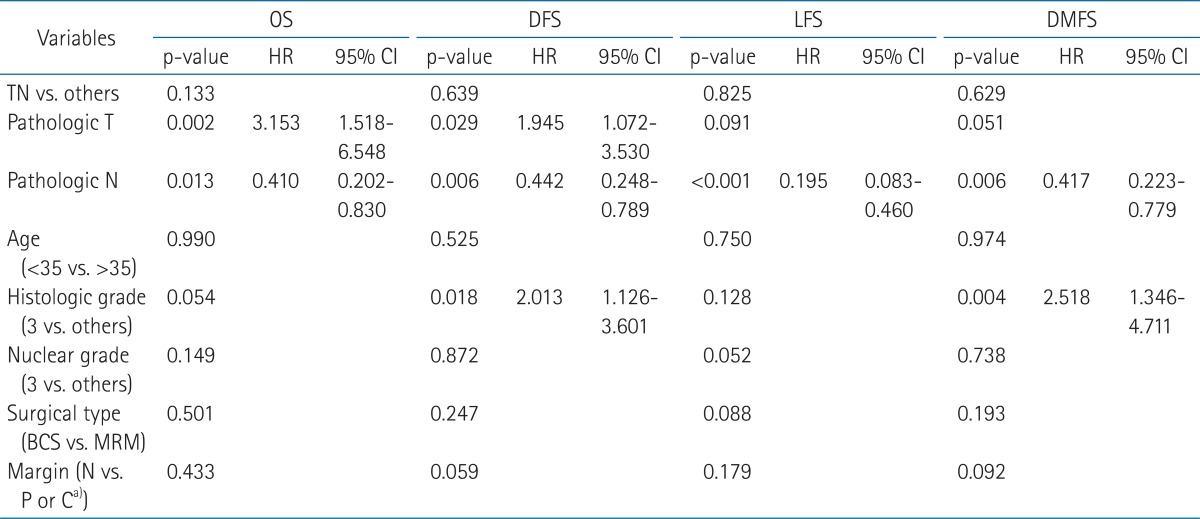
Of the 446 patients diagnosed with early breast cancer, in whom all three markers (ER, PR, and HER-2) were available, 94 (21.1%) were classified as TN, 57 (12.8%) as HER-2 type, and 295 (66.1%) as luminal. Characteristics of the patients according to molecular subtype are summarized in Table 1. Young patients younger than 35 years old were more distributed in TN type (14.9%) compared with HER-2 (8.8%), and luminal (7.1%, p = 0.002). The distribution of histology was different among three molecular groups. HER-2 type and luminal had more ductal carcinoma than TN (p = 0.0001). There was a substantial difference in the overall distribution of histologic grade and nuclear among three molecular groups. TN type was more frequently associated with higher histologic grade (p < 0.0001), and nuclear (p < 0.0001). The distribution of a surgical method was not different among three groups (p = 0.066). The distribution of pathologic T stage, N, resection margin, and administration of adjuvant chemotherapy were not different among three groups.
2. Treatment outcomes
With a median follow-up period of 78 months (range, 4 to 130 months) in this analysis, there were 9 local relapses (2.0%), 15 nodal relapses (3.4%), 40 distant metastases (9.0%) and 33 deaths (7.4%) for all patients (Fig. 1). For patients in the luminal group, local, regional recurrence was 1.4%, 3.1% compared with 1.8%, 5.3% for HER-2 group, and 3.2%, 2.1% for TN group, respectively. For patients in the luminal group distant recurrence was 7.8% compared with 10.5% for HER-2, and 11.7% for TN group, respectively.
The rates of 5-year OS, DFS, LFS, and DMFS for all patients were 95.5%, 89.9%, 95.4%, and 91.7%, respectively.
The rates of OS, DFS, LFS, and DMFS were not significantly different between TN type and luminal, TN and HER-2, or between TN and other molecular subtypes (p > 0.05) (Fig. 2, Table 2). For patients in the TN type, the rates of 5-year OS, DFS, LFS, and DMFS were 94.2%, 87.0%, 94.6%, 87.9% compared with 92.2%, 85.0%, 92.4%, and 90.4% for HER-2, and 96.5%, 91.7%, 96.2%, and 93.1% for luminal, respectively (Table 2). Univariate survival analysis revealed that pathologic T stage, N, and nuclear grade were significant prognostic factors for 5-year OS, DFS, LFS, and DMFS (p < 0.05). Histologic grade was significant for 5-year OS, DFS, and DMFS and positive or close margin was significant for DFS, LFS, and DMFS (p < 0.05) (Table 2).

The rates of overall survival (A), disease free survival (B), locoregional free survival (C), distant metastasis free survival (D) between triple negative (TN) type and human epidermal growth factor receptor 2 (HER-2) or luminal.
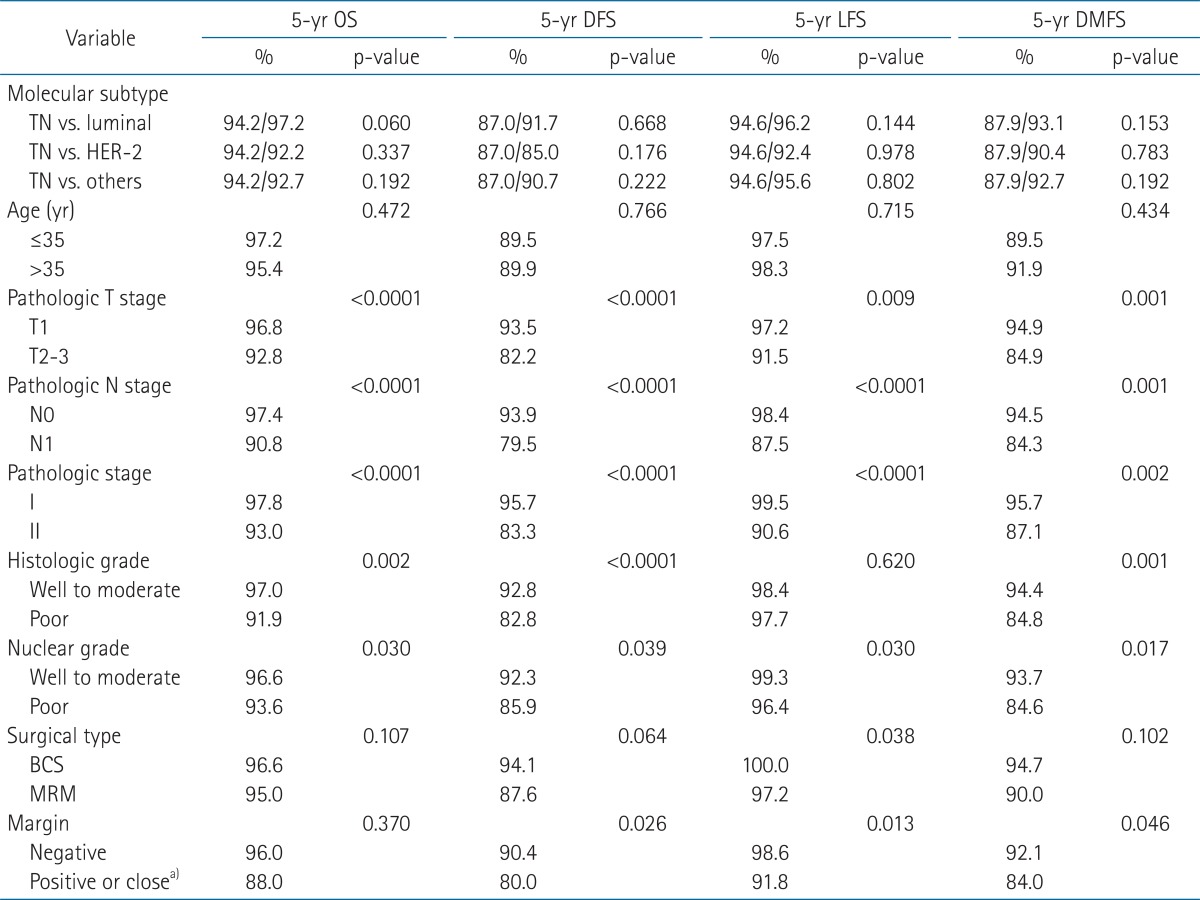
Five-year overall survival (OS), disease-free survival (DFS), locoregional relapse-free survival (LFS), and distant metastasis-free survival (DMFS) for all patients (n = 446) using log-rank test
Multivariable Cox analysis for OS, DFS, LFS, and DMFS revealed that pathologic N stage was significant prognostic factor for OS, DFS, LFS, and DMFS and pathologic T for OS, and DFS. Histologic grade was prognostic factor for DFS and DMFS (Table 3).
Discussion and Conclusion
In the current study, we evaluated 446 patients with early stage breast cancer in whom all three markers were available to determine whether TN early stage breast cancers have poorer survival rates compared with other molecular subtypes. In agreement with previous studies, in our series, TN type was associated with higher histologic grade, nuclear grades, and younger patient distribution [5,14]. However, in our results, we found that early stage patients with TN molecular subtype did not seem to have poorer survival rates compared with HER-2 type or luminal.
Because patients with TN type breast cancer do not benefit from endocrine therapy or trastuzumab, it reflects the intrinsically adverse prognosis. Several studies have demonstrated that TN type breast cancers are associated with a poor prognosis [5,8,10]. Dent et al. [8] investigated clinical features and patterns of recurrence of 180 patients with TN type and compared it with 1,421 with other molecular subtypes. In their study [8], patients with TN type had an increased likelihood of distant recurrence (33.9% vs. 20.4%, respectively; p < 0.0001), an increased death within 5 years (42.2% vs. 28%, respectively; p < 0.0001), and shorter median time to death. Voduc et al. [10] analyzed 2,985 patients with breast cancers according to molecular subtype and demonstrated increased locoregional recurrence (p < 0.001) of TN breast cancer.
However, the treatment results in patients with TN early stage breast cancers are not consistent. Nguyen et al. [5] investigated 793 patients with invasive breast cancer who received BCS, based on the three markers; luminal A and B, basal, and HER-2, and demonstrated that basal subtypes were associated with increased local recurrence and distant metastasis. On the contrary, Haffty et al. [11] and Freedman et al. [15] reported that isolated locoregional relapse was not increased in patients with TN type after BCS. In another study, Solin et al. [12] investigated 519 patients with early stage breast cancers treated with BCS, and indicated that patients with TN type had a higher rate of local failure compared with other types but lower rate of distant recurrence (p = 0.0006). In recent study, furthermore, Noh et al. [6] demonstrated that the pattern of recurrence of locoregional and distant metastasis was not different according to molecular subtypes in patients with early stage breast cancer after BCS.
Differences in the pathologic T stage and N included in the previous studies [5,6,11,12,15] could partially explain the different treatment results. Nguyen et al. [5] included patients with pathologic T1 to T3, and N0 to N3. Solin et al. [12] and Freedman et al. [15] included patients with pathologic T1 to T2, and N0 to N3, and Noh et al. [6] included only with pathologic T1 to T2, and N0 to N1. In the current study, we analyzed patients with pathologic I, II disease, and in agreement with Noh et al. [6], indicated that the prognosis of TN early stage breast cancer was not poorer than other molecular subtypes.
TN type breast cancer had not been well recognized until 2005 [3,16], since then, the term has appeared in a PubMed search of the medical literature and there are no prospective studies of its treatment outcomes. Recently, two studies [14,17] suggested that TN breast cancers might have different biology and clinical behaviors. Park et al. [14] assessed whether the TNM staging system could function as an independent prognostic indicator for predicting long-term clinical outcomes for each molecular subtype in 1,879 patients with breast cancer. Interestingly, there was no relationship between TNM staging and clinical outcome in terms of relapse free survival and distant recurrence free survival in TN breast cancers of stage 1 to 3a unlike other molecular types, indicating that TN early stage breast cancers are barely influenced by stage [14]. Kim et al. [17] analyzed prognostic significance of young age in 2,474 patients by subtype based on ER, PR, and HER-2 status in breast cancer and demonstrated that patients younger than 35 years old was not a poor prognosticator for recurrence and cancer-specific survival in TN subtype (p = 0.242) unlike other subtypes (p < 0.001), indicating that young age factors are not adapted in TN type breast cancers. Therefore, in the future it will be necessary to study prospective trials of clinical behaviors and biologic characteristics for a prognosis in patients with TN early stage breast cancer.
Although there is general agreement that the majority of TN breast cancers can be categorized as basal-like, incomplete TN definition is a potential weakness of the present study. Another weakness of the study is the limitation possessing potential biases imposed by retrospective analysis and relatively small sample size. For the current study, because of small sample size, treatment outcomes were not analyzed in homogeneous surgical group, however there was no difference of distribution in surgical methods among the three molecular subtypes and there was no difference for treatment outcomes according to MRM or BCS. Another limit of this study is that HER-2 data was obtained through immunohistochemical testing not by FISH. Different chemotherapy regimen might influence the treatment outcome of the present study and since the current study was analyzed in patients before trastuzumab era, the results in patients treated with trastuzumab in HER-2 type could be different.
In, conclusion, we evaluated whether TN early stage breast cancers have poorer survival rates compared to other molecular types. Although further follow-up needs, there was no difference in OS, DFS, LFS, and DMFS compared with other molecular subtypes. In the near future, prospective study in homogeneous treatment group will need to predict a prognosis in patients with TN early stage breast cancer.
Notes
No potential conflict of interest relevant to this article was reported.