Comparison study of intensity modulated arc therapy using single or multiple arcs to intensity modulated radiation therapy for high-risk prostate cancer
Article information
Abstract
Purpose
Intensity modulated arc therapy (IMAT) is a form of intensity modulated radiation therapy (IMRT) that delivers dose in single or multiple arcs. We compared IMRT plans versus single-arc field (1ARC) and multi-arc fields (3ARC) IMAT plans in high-risk prostate cancer.
Materials and Methods
Sixteen patients were studied. Prostate (PTVP), right pelvic (PTVRtLN) and left pelvic lymph nodes (PTVLtLN), and organs at risk were contoured. PTVP, PTVRtLN, and PTVLtLN received 50.40 Gy followed by a boost to PTVB of 28.80 Gy. Three plans were per patient generated: IMRT, 1ARC, and 3ARC. We recorded the dose to the PTV, the mean dose (DMEAN) to the organs at risk, and volume covered by the 50% isodose. Efficiency was evaluated by monitor units (MU) and beam on time (BOT). Conformity index (CI), Paddick gradient index, and homogeneity index (HI) were also calculated.
Results
Average Radiation Therapy Oncology Group CI was 1.17, 1.20, and 1.15 for IMRT, 1ARC, and 3ARC, respectively. The plans' HI were within 1% of each other. The DMEAN of bladder was within 2% of each other. The rectum DMEAN in IMRT plans was 10% lower dose than the arc plans (p < 0.0001). The GI of the 3ARC was superior to IMRT by 27.4% (p = 0.006). The average MU was highest in the IMRT plans (1686) versus 1ARC (575) versus 3ARC (1079). The average BOT was 6 minutes for IMRT compared to 1.3 and 2.9 for 1ARC and 3ARC IMAT (p < 0.05).
Conclusion
For high-risk prostate cancer, IMAT may offer a favorable dose gradient profile, conformity, MU and BOT compared to IMRT.
Introduction
Prostate cancer is a site in which highly conformal therapies have gained widespread use secondary to the organs at risk in the area and the benefit seen with dose escalation in meta-analysis [1]. In many large centers, intensity modulated radiation therapy (IMRT)-based plans have become standard for patients with prostate cancer. Volumetric modulated arc therapy (VMAT) is a specific form of IMRT, which has recently gained popularity. This technique delivers radiation dose with up to 360-degree gantry rotations while the multileaf collimators (MLCs) transition at various angles. The radiation can be delivered at a constant dose rate (cdr-VMAT) or a variable dose rate (vdr-VMAT). Arc therapy can be delivered as multiple or single arcs. Single arc-modulated radiation therapy (AMRT) delivers dose in a single gantry rotation and multi-arc intensity-modulated arc therapy (IMAT) uses multiple overlapping arcs. The advantage of IMAT is its highly conformal dose distribution [2], whereas AMRT is more efficient, delivering the same dose in a shorter period of time [3].
In this study we compare IMRT, to 1ARC, and 3ARC IMAT (RapidArc; Varian Medical Systems, Palo Alto, CA, USA) to address these issues. Specifically, we studied sixteen patients with high-risk prostate cancer as defined by the National Comprehensive Cancer Network (NCCN). Our goal is to quantify the target volume adequacy of coverage and efficiency as well as dose to organs at risk (OARs). Additionally, we compare results from low dose volume of radiation for each modality.
Materials and Methods
Sixteen patients with high-risk prostate cancer (prostate-specific antigen [PSA] > 20 and/or Gleason score ≥ 8) were included in this study. The study was approved by our Institutional Review Board. Computed tomography (CT) scan simulation was acquired for all patients and the images were imported to the Eclipse treatment planning station (ver. 8.6, Varian Medical Systems). The radiation oncologist contoured the prostate and seminal vesicles (CTVP) as well as the pelvic lymph nodes based on CT images. The corresponding PTVs were created by adding a 1-cm margin around the pelvic lymph nodes in all directions, and 0.7 cm around the CTVP in all directions except the posterior margin where a 0.5 cm margin was used. A PTVB was also created by expanding the CTVP by 0.6 cm in all directions except the posterior margin where a 0.5 cm margin was used.
Rectum, bladder, small bowel, and acetabulum were contoured as the OARs. The rectum was contoured starting at the level of ischial tuberosities to the rectosigmoid junction. The urinary bladder was contoured based on the CT simulation images. Similarly, the small bowel loops were contoured based on the CT images to 3 cm superior to the top slice of the PTVP. Total planned dose was 79.20 Gy/1.8 Gy/fraction. The PTVRtLN, PTVLtLN, and initial PTVP received 50.4 Gy/1.8 Gy/fraction followed by a 1ARC boost of 28.80 Gy to PTVB. This study reports only on the initial dose of 50.40 Gy to the first 3 PTVs because of the inclusion of the lymph nodes in the target volume and the theoretical benefit of the arc-modulated therapy in this setting.
Data were collected and summarized. Two-sided sign tests have been conducted to compare between the three plans, two at a time (Table 1), p-value of <0.05 is considered significant. STATA statistical software package ver. 12.0 (STATA Corp., College Station, TX, USA) has been used for the analysis.
1. Treatment planning
All treatments were planned using the Eclipse treatment planning system, and delivered by the iX linear accelerator (Varian Medical Systems). Three plans were generated per patient, as illustrated in (Fig. 1).
1) An IMRT plan with one isocenter and 7 static fields. The fields were evenly distributed every 51.5°, starting from the anterior field. To minimize the dose to the rectum, the lower two posterior oblique fields were designed to spare the organ by partially covering the target, and they had a fixed size during the optimization. The MLC motion was optimized using the sliding window technique, resulting in a slightly higher number of monitor units (MUs) and a significantly lower beam on time (BOT). The dose rate was equal to 300 MU/min.
2) A one field ARC plan, with the beam performing a full 360° rotation around the single isocenter. The gantry rotated from 179.9° to 180.1°. The dose rate was variable with a maximum value of 600 MU/min (averaging around 300 MU/min).
3) A three coplanar fields ARC plan. Each field had a separate isocenter, each one located at the center of the corresponding PTV. The gantry performed a full 360° rotation around PTVP, and a partial 200° rotation around PTVRtLN (clockwise 200° to 40°) and PTVLtLN (counter clockwise 40° to 200°). The dose rate was variable with a maximum value of 600 MU/min (averaging around 300 MU/min).
All plans were optimized using a standard planning constraint set based on the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) [4] and Radiation Therapy Oncology Group (RTOG) protocols [5], dose objectives and priorities. All three plans maintained the maximum dose DMAX ≤110%, and were normalized to ensure 95% coverage for the total PTVT (PTVP + PTVRtLN + PTVLtLN).
2. Plan evaluation
Plans were evaluated in terms of quality and efficiency. For this purpose, we recorded the monitor units, beam on time, the mean dose (DMEAN) to the OARs, and the volume covered by the 50% isodose line (V50).
Treatment efficiency was evaluated based on the comparison of the:
- MU defined as the average number of monitor units to required deliver the prescribed dose.
- BOT defined as the time the treatment planning system (Eclipse, Varian Medical Systems) predicts the beam will be "on" to deliver the prescribed monitor units. Does not account for time required to reach each gantry position; as in the case of IMRT.
Treatment quality included the comparison of the mean dose (DMEAN) to the OAR such as the bladder and the rectum, the volume covered by the 50% isodose line (V50), and the following indexes:
- Conformity index (CI) calculated according to the RTOG index score definition: CI = PV95%/PTV, where PV95% is the volume that received the effective prescribed dose (95% in this study), and PTV is the planned treatment volume.
- Paddick gradient index (GI), defined as CI = PV50%/PV, where PV50% is the volume that received 50% of the effective prescribed dose, and PV the prescribed dose (PV95% in the study).
- Homogeneity index (HI) calculated as HI = IMAX/RI, where IMAX is the maximum isodose in the target and RI is the reference isodose (100% in this study).
For each one of the above parameters, the average value and the standard deviation (SD) were calculated. Plans were reviewed for dose distribution, OAR sparing, and integral dose to surrounding normal tissue. All of them satisfied the planning objectives, and considered acceptable for treatment.
Results
1. Plan efficiency
BOT: The BOT decreased with the IMAT plans compared to IMRT. The shortest time was achieved with 1ARC. The average BOT for IMRT, 1ARC and 3ARC plans were 6.0, 1.4, and 3.2 minutes, respectively (Fig. 2A). The range (and SD) was 4.75-7.55 (0.75), 1.25-1.86 (0.28), and 2.63-3.59 (1.03), respectively.

(A) Average "Beam On" time in minutes and (B) average number of monitor units (MU). IMRT, intensity modulated radiation therapy.
MU: The MU decreased significantly with the IMAT plans compared to IMRT; lowest number was achieved with 1ARC. The average MU was 1,739, 576, and 1,086 for IMRT, 1ARC, and 3ARC plans, respectively (Fig. 2B). The range (SD) was 956-2,260 (264), 481-648 (51), and 644-1,345 (166), respectively.
2. Plan quality
Mean dose (DMEAN) for OAR: Rectal DMEAN was lower with IMRT in comparison to IMAT plans and was comparable between 1ARC and 3ARCs. There was no significant difference between the three plans as far as the DMEAN of bladder (Fig. 3).
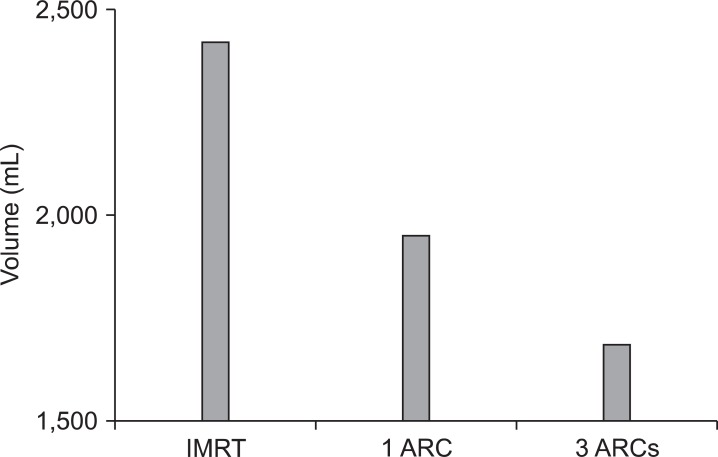
Integral dose (V50): The volume covered by the 50% isodose line was significantly higher with the IMRT plan in comparison to the IMAT plans. The average V50 (in mL) among the three plans was 2,423, 1,952, and 1,685 for IMRT, 1ARC, and 3ARC, respectively (Fig. 4).
CI: The CI was comparable among the plans. The average CI among the three plans was 1.16, 1.19, and 1.15, respectively (Fig. 5). The 1ARC plan was significantly better than IMRT (p = 0.0352), none of the other plans were significant (NS).
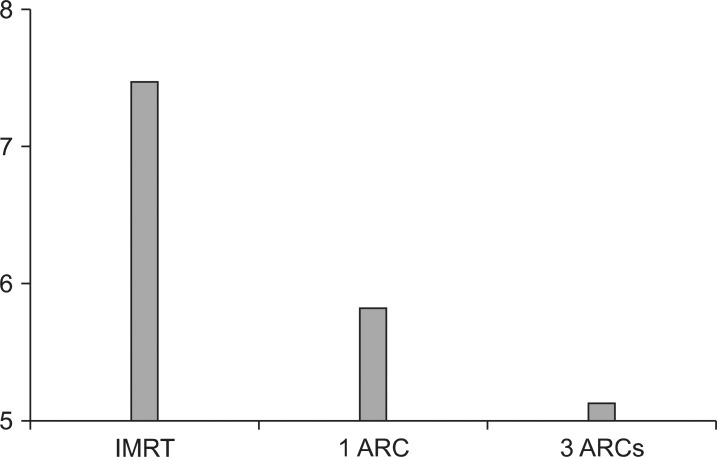
GI: The average GI was 7.54, 5.93, and 5.24 for IMRT, 1ARC, and 3ARC plans, respectively (Fig. 6). There improvement was significant when using IMAT compared to IMRT. It was also decreased significantly when 3ARC was compared to 1ARC.
HI: There was no significant difference amongst the three plans in terms of the HI (Fig. 7).
For the aforementioned variables, the statistical significance (based on the p-value score) of the difference between the plans, is summarized in Table 1.
Discussion and Conclusion
Prostate cancer is a site in which highly conformal therapies have gained widespread use secondary to the organs at risk in the area and the benefit seen with dose escalation in meta-analysis [1]. In many large centers, IMRT-based plans have become standard for patients with prostate cancer. Although treatment volumes for those patients affected with low and intermediate risk prostate cancer have reached a general consensus, treatment of high-risk prostate cancer continues to be a controversial topic. The debate regarding the radiation therapy field is still ongoing and the inclusion of the pelvic lymph nodes remains. Radiation toxicity in those patients receiving high-doses to both the prostate and pelvic lymph nodes has been noted with even lower doses than currently used [2]. We studied sixteen patients with high-risk prostate cancer as defined by NCCN to evaluate the target volume adequacy of coverage and efficiency as well as dose to OARs.
IMRT has been shown to improve coverage of the CTV by the prescription dose and reduced the volumes of the rectal and bladder walls significantly when compared to 3D conformal radiation therapy techniques in prostate cancer [3]. These benefits led to the widespread use of modulated therapy in prostate cancer and lead to the current standard of dose escalation [6]. VMAT represents a specific type of IMRT that was conceived to exploit increasing number of fields and decreasing treatment time [7,8]. By constantly changing MLC position, gantry position, and dose rate, VMAT has been shown to deliver equivalent plans in shorter time when compared with IMRT [9].
VMAT has been proposed to result in reduced treatment times and monitor units when compared to IMRT [10]. It has been shown to decrease the number of MUs required [11]. There has been a hypothesized benefit in reducing secondary malignancies due to the reduction of interleaf scatter [12]. Additionally, arc therapy with multiple arcs allows for flexibility of dosage, increased sparing of normal tissue, and increased conformality [13,14]. VMAT, specifically multiple arcs, has been shown in head and neck cancer to provide better PTV dose homogeneity and similar or better OAR sparing [15,16]. VMAT, specifically multiple arcs, has also been shown to provide comparable PTV coverage in spine stereotactic body radiation therapy with greater efficiency compared to IMRT [17]. Enhanced sparing of the rectum has been achieved with VMAT in prostate cancer patients undergoing dose escalation to an intraprostatic lesion [18].
We investigated the feasibility of using multiple static fields IMRT to single arc and 3 arcs IMAT in patients with high-risk prostate cancer. All three plans were comparable as far as adequacy of coverage. We chose the RTOG CI to report on the target volume coverage. The conformity indices were 1.17, 1.20, and 1.15 for IMRT, 1ARC, and 3ARC, respectively; with 3ARC exhibiting slightly higher conformity. The conformity indices reported by Yoo et al. [19] and associates were 1.19, 1.25, and 1.20 for IMRT, single arc, and 2 arcs, respectively. Arc therapy was significantly better than IMRT but no significant difference among the one versus 2 arcs. The GI, or "dose fall off" measure, also revealed an improvement with arc therapy in comparison to IMRT plans. V50-the volume covered by the 50% isodose line was significantly higher with the IMRT plan in comparison to the IMAT. DMEAN for organs at risk was lower with IMRT in comparison to IMAT plans and was comparable between 1ARC and 3ARC. There was no significant difference between the three plans as far as the DMEAN of bladder. Our results are suggesting that VMAT offer more conformal plans in high-risk prostate cancer patients with minimal change in dose to organs at risk.
Efficiency of treatment was also improved in our cohort of patients when comparing IMRT versus arc therapy, with reductions in "Beam ON" time and number of MU. The average BOT decreased 4.6 and 2.8 minutes with the single arc and multiple arcs IMAT, respectively, plans compared to IMRT. The benefits of reduced "Beam ON" time include faster treatment time for the patient, which may result in greater patient comfort and increased number of patients to be treated on a machine. Also, with treatment given in a shorter amount of time, probability of intrafractional movement decreases. Average MU decreased significantly with the IMAT plans compared to IMRT; lowest number was achieved with 1ARC.
VMAT appears to improve treatment efficiency, dosimetry, and conformity for patients with high-risk prostate cancer when compared to IMRT. This could translate into increased patient quality of life and linear accelerator productivity.
Notes
Presented at the American Society of Therapeutic Radiation Oncology (ASTRO) meeting 2010, San Diego, CA, S362.
No potential conflict of interest relevant to this article was reported.