Esophageal tolerance to high-dose stereotactic radiosurgery
Article information
Abstract
Purpose
Esophageal tolerance is needed to guide the safe administration of stereotactic radiosurgery (SRS). We evaluated comprehensive dose-volume parameters of acute esophageal toxicity in patients with spinal metastasis treated with SRS.
Materials and Methods
From May 2008 to May 2011, 30 cases in 27 patients with spinal metastasis received single fraction SRS to targets neighboring esophagus. Endpoints evaluated include length (mm), volume (mL), maximal dose (Gy), and series of dose-volume thresholds from the dose-volume histogram (volume of the organ treated beyond a threshold dose).
Results
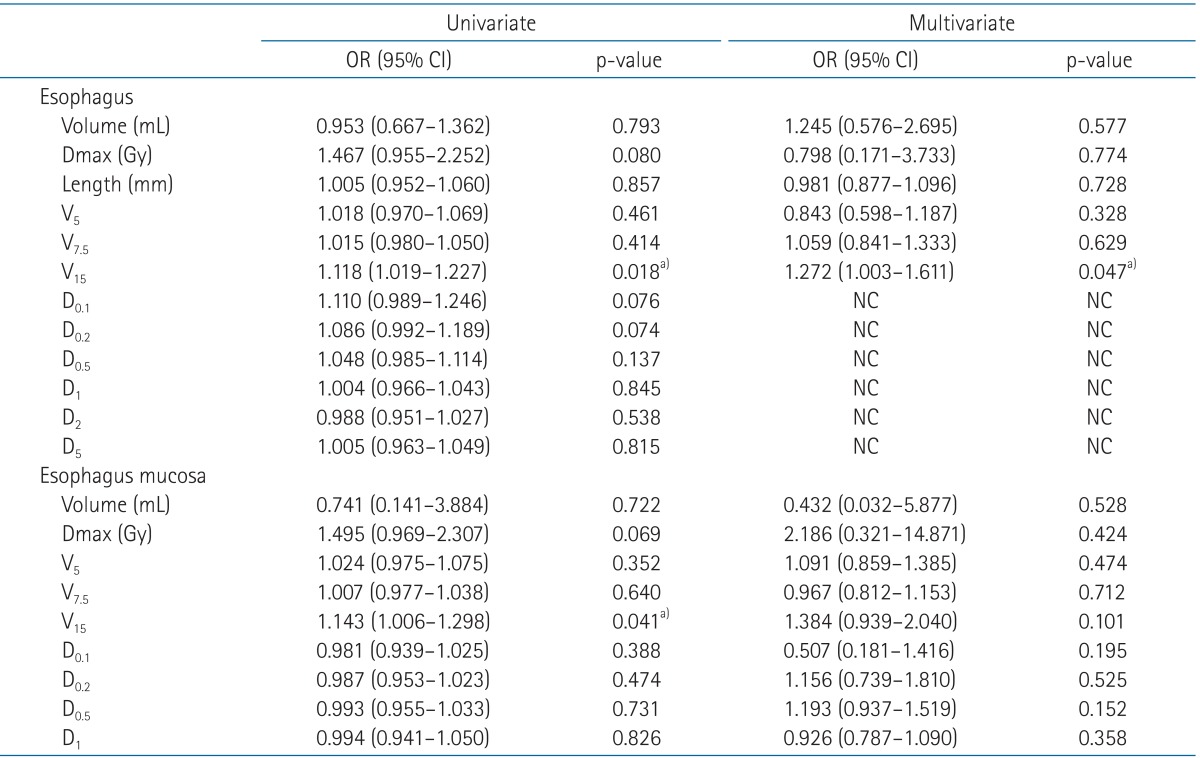
The median time from the start of irradiation to development of esophageal toxicity was 2 weeks (range, 1 to 12 weeks). Six events of grade 1 esophageal toxicity occurred. No grade 2 or higher events were observed. V15 of external surface of esophagus was found to predict acute esophageal toxicity revealed by multivariate analysis (odds radio = 1.272, p = 0.047).
Conclusion
In patients with spinal metastasis who received SRS for palliation of symptoms, the threshold dose-volume parameter associated with acute esophageal toxicity was found to be V15 of external surface of esophagus. Restrict V15 to external surface of esophagus as low as possible might be safe and feasible in radiosurgery.
Introduction
Acute esophageal toxicity is a common side effect of patients undergoing spinal stereotactic radiosurgery (SRS). However, relative paucity of the information regarding clinical and dosimetric predictors of esophageal toxicity in SRS is known and whether predictions using conventional fractionation could be applied to SRS is uncertain. Although numerous reports have been published on serious esophageal toxicity from conventional fractionation, comprehensive dose-volume-based analyses from a single-fraction radiosurgery was not extensively studied.
We herein assess correlations between acute esophageal toxicity in patients treated with SRS and either absolute irradiated length (mm), volume (mL), maximal dose (Gy), and series of dose-volume thresholds from the dose volume histogram (DVH; volume of the organ treated beyond a threshold dose) in one institution.
Materials and Methods
Thirty cases of 27 patients with C3-T10 spinal metastases received spinal radiosurgery between May 2008 and May 2011. Table 1 provides a summary of patient characteristics. Treatment was designed to provide a single fraction of 16 to 22 Gy (median, 19 Gy) prescribed to the 90% isodose line encompassing the planning target volume (PTV) planned by intensity-modulated radiotherapy except one case. In the present study, patients were treated with a Novalis radiosurgery system (Brainlab AG, Heimstetten, Germany).
Acute esophageal toxicity occurs in second to third weeks after initiation of radiation therapy (RT), manifested as dysphagia and substernal discomfort. RT limits the proliferative ability of the epithelium, which progress to denudation causing thinning of the mucosa [1]. Because the paper dealt with acute esophagitis, we considered esophagus mucosa in target delineation in addition to esophagus. Esophagus was contoured based on outer esophageal wall which is external surface, wheareas esophagus mucosa contour was based on inner esophageal epithelial layer, depicted in Fig. 1. They were retrospectively contoured by single author on the same treatment planning axial computed tomography (CT) images with 2 mm-thick slices from cricoid cartilage superiorly to gastro-esophageal junction inferiorly using a mediastinal window setting.
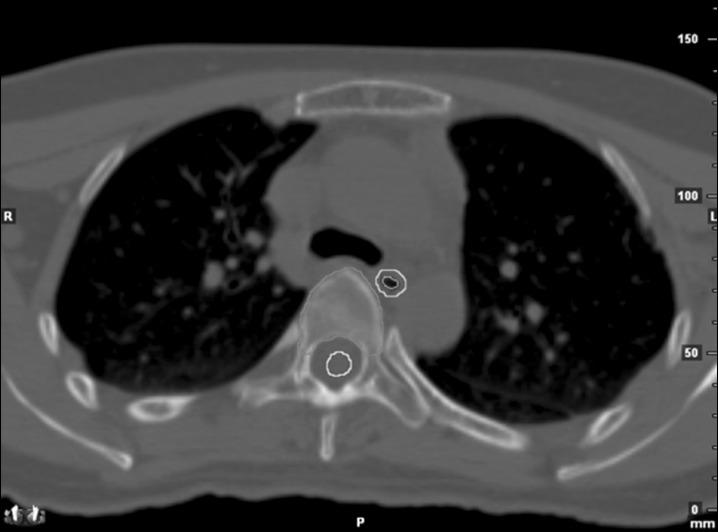
Green line indicates external surface of esophagus, orange line indicates internal mucosa of esophagus, and yellow line indicates spinal cord.
Esophageal toxicity was assessed by review of treatment charts, graded by the Radiation Therapy Oncology Group (RTOG) criteria (Table 2). Dose-volume constraints of lung and spinal cord were listed in Table 3. Spinal magnetic resonance imaging (MRI) was conducted at baseline. Follow-up MRI was not performed routinely in all patients but was performed in those patients with clinical evidence of progression. The median follow-up for the 27 patients were 5 months (range, 0 to 26 months).
The following dosimetric parameters were extracted from the 3-dimensional dose data set: absolute volume (mL) and length (mm), maximal dose (Gy) of esophagus and esophagus mucosa irradiated, percent of volume of esophagus and esophagus mucosa receiving 5, 7.5, and 15 Gy (V5, V7.5, and V15) and absolute dose irradiated to 0.1, 0.2, 0.5, 1, 2, and 5 mL (D0.1, D0.2, D0.5, D1, D2, and D5) of esophagus and esophagus mucosa. Length and volume of PTV plus an additional 6 mm were considered as length and volume of esophagus and esophagus mucosa. Length of esophagus mucosa was not taken into account, for its length equals to that of esophagus.
SPSS ver. 19.0 (IBM, Armonk, NY, USA) statistic package program was used to identify which of dosimetric variables could predict for the occurrence of acute esophageal toxicity. All analyses were two-sided. Student t-test as a parametric statistical hypothesis and Mann-Whitney test as a non-parametric statistical hypothesis test were performed to evaluate differences in mean values between acute esophageal toxicity group (group 1) and non-acute esophageal toxicity group (group 2). Univariate and multivariate logistic regression analysis were performed to find out the significant predictor of acute esophageal toxicity. Finally the receiver operating characteristic (ROC) graphs were performed to find out critical starting cutoff value causing acute esophagitis.
Results
Acute esophageal toxicity of grade 1 occurred in 6 (20%) of the 30 cases in 27 patients. The median time from the start of irradiation to development of esophageal toxicity was 2 weeks (range, 1 to 12 weeks). No grade 2 or higher events were noticed. Maximal dose (Dmax) of esophagus mucosa of group 1 was found to be statistically significantly different from that of group 2 in Student t-test (p = 0.046; p < 0.05), but means were not statistically significantly different by Mann-Whitney test with p = 0.062 (p > 0.05). However, D0.1 and D0.2 of esophagus in group 1 were found to be statistically significantly different from those in group 2 (p = 0.002 and p = 0.008, respectively).
Univariate and multivariate logistic regression analysis were carried out with the description of variables on Table 4. In univariate logistic regression analysis, group 1 has 1.118 and 1.143 odds as group 2 at V15 of esophagus (p = 0.018; p < 0.05) and V15 of esophagus mucosa (p = 0.041; p < 0.05), respectively. D0.1, D0.2, D0.5, D1, D2, and D5 of esophagus were impossible to be calculated in multivariate logistic regression analysis for their odds ratio were not established. Other variables were found not to be statistically significant. However, V15 of esophagus was the only significant predictor of acute esophageal toxicity revealed by multivariate logistic regression analysis (odds radio = 1.272, p = 0.047). The ROC graphs were performed to find out critical starting cut off value causing acute esophagitis. The area under ROC curve of V15 was 0.812 with a cutoff value of 0.104 mL, the sensitivity was 100% and the specificity was 54.2%. Therefore, dose of 15 Gy to esophagus as small as 0.104 mL, so far as to be a critical threshold value, would prevent acute esophagitis in patients treated with SRS in this report.
Discussion and Conclusion
The clinical applications of radiosurgery to spinal metastasis have been rapidly evolving. While efforts have focused on evaluating spinal cord tolerance, few recommendations exist for tolerance of adjacent esophagus [2].
Viewed from conventional fractionation RT, where acute esophagitis events occur mainly during a course of therapy, rapidity of dose accumulation might be more important than the final overall dose [3]. SRS is highly rapid treatment modality, and without any constraints of esophagus, radiation dose could be dumped inadvertently. Therefore, dose limitation of esophagus in radiosurgery should be specified.
The esophagus is mobile organ. Dieleman et al. [4] reported intrafractional mobility could be significant, particularly for the distal esophagus. Average of the absolute interfraction and intrafraction displacement in esophageal motion during radiotherapy was reported to be 4.2 mm or less [5]. Possible systemic error cannot be avoided in the present study due to challenging defining external surface and internal lumen of esophagus and its genuine mobility. From this point of view, much care in delineating esophagus is required in SRS than conventional RT because high fractional dose can be administered to error-contoured, especially sized-down, esophagus.
The DVH analysis is commonly used in RT planning to evaluate toxicity of normal tissues included in RT fields [6]. However, standard DVH were suggested not to convey the information regarding does distribution along the esophageal circumference that might be a useful predictor of toxicity for any tubular organ [6-9]. In the study of Kahn et al. [10], esophagus does not apparent in CTs, likely because of its folds and undulations, which demonstrate variability in esophageal areas of approximately 31%, with a range of 14% to 77%, suggesting that DVH based dosimetric parameters typically rely on CT-defined esophageal contours, and thus systematic limitations in esophageal contouring will influence these parameters. However, examining the DVH parameters by contouring both external and internal lumen of esophagus in this paper, suggests various data sets more informative and reliable.
Currently a few reports have been published of serious esophageal injury from single fraction stereotactic body radiation therapy (SBRT), with little comprehensive dose-volume based analyses to identify a best threshold volumetric parameter for esophageal irradiation. The guidelines of dose constraints for the esophagus in single-fraction SBRT in Memorial Sloan-Kettering Cancer Center were as follows: level 1 dose constraint of esophagus is 15 Gy/2 mL, while level 2 is 20 Gy/2 mL [11], which are consistent with our findings. Timmerman [12] recommended dose constraints of esophagus for single-fraction SBRT to be 14.5 Gy to less than 5 mL (endpoint ≥ grade 3). In RTOG 0915 clinical trial of randomized phase II study comparing 2 SBRT schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer, they restrict esophageal Dmax to 15.4 Gy and 5 mL (D5) to 11.9 Gy in single-fraction SBRT. In RTOG 0631 trial of phase II/III study of image-guided radiosurgery/SBRT for localized spine metastasis, esophagus volume less than 0.03 mL irradiated up to 16 Gy or 5 mL to 11 Gy are recommended for one fraction dose constraints.
The study has found V15 of external surface of esophagus to be dose-volume parameter associated with acute esophageal toxicity. Although grade 1 esophageal toxicity might not be grave and manageable in long standpoint of care, with parameter associated with acute esophageal toxicity as V15, immediate discomfort right after treatment could be expected by clinicians and directed to patients ahead.
The present study contains weaknesses. It is retrospective study, and the results are based on marked small and heterogeneous patient population, limited to generalize our results. Furthermore the low rate of complications restricted the strength to perform reliable statistics to retrieve better dose constraints. To confirm the conclusion, further prospective trials with large cases might be necessary.
In patients with spinal metastasis who received SRS for palliation of symptoms, the threshold dose-volume parameter associated with acute esophageal toxicity was found to be V15 of external surface of esophagus. Restrict V15 to external surface of esophagus as low as possible might be safe and feasible in radiosurgery.
Notes
No potential conflict of interest relevant to this article was reported.