Chemoradiotherapy in squamous cell carcinoma of the anal canal: a single institution experience
Article information
Abstract
Purpose
We reviewed the treatment outcomes and prognostic factors for patients with anal canal carcinoma who were treated with curative intent chemoradiotherapy (CRT) at Severance Hospital from 2005 to 2011.
Materials and Methods
Data for 38 eligible patients treated during this period were reviewed. All patients were treated with curative intent using radiotherapy (RT) with (n = 35) or without concomitant chemotherapy (n = 3). Among 35 patients who received CRT, most of the chemotherapeutic regimens were either 5-fluorouracil (5-FU) plus mitomycin C (23 patients) or 5-FU plus cisplatin (10 patients). Recurrence-free survival (RFS), colostomy-free survival (CFS), overall survival (OS), and locoregional control (LRC) rates were calculated using the Kaplan-Meier method and survival between subgroups were compared using the log-rank test. Cox's proportional hazard model was used for multivariate analysis.
Results
Over a median follow-up period of 44 months (range, 11 to 96 months), 3-year RFS, CFS, OS, and LRC were 80%, 79%, 85%, and 92%, respectively. In multivariate analysis, tumor size >4 cm was an independent predicting factor for poorer RFS (hazard ratio [HR], 6.35; 95% confidence interval [CI], 1.42 to 28.5; p = 0.006) and CFS (HR, 6.25; 95% CI, 1.39-28.0; p = 0.017), while the presence of external iliac lymph node metastasis was an independent prognosticator for poorer OS (HR, 9.32; 95% CI, 1.24 to 70.3; p = 0.030). No treatment-related colostomies or deaths occurred during or after treatment.
Conclusion
Curative intent CRT resulted in excellent outcomes that were comparable to outcomes in previous randomized trials. No severe treatment-related toxicities were observed.
Introduction
Squamous cell carcinoma of the anal canal is an uncommon malignancy [1], representing only 0.1% of all malignancies in Korea [2]. Two phase III trials begun in the 1980s established that the combination of 5-fluorouracil (5-FU) and mitomycin C (MMC) with radiotherapy (RT) improves tumor control [3,4]. The Radiation Therapy Oncology Group (RTOG) 87-04 trial subsequently confirmed the superiority of 5-FU + MMC over 5-FU alone when combined with RT. Results of the RTOG 98-11 trial and the Anal Cancer Trial (ACT) II indicate no advantage of 5-FU + cisplatin (CDDP) compared to 5-FU + MMC administered during chemoradiotherapy (CRT) [5,6]. The RTOG 98-11 and the ACCORD-03 trials tested CDDP-based neoadjuvant chemotherapy (NACT) and failed to show improvement in either disease-free survival (DFS) or colostomy-free survival (CFS) [5,7]. The long-term results of the RTOG 98-11 trial show significantly better survival for 5-FU + MMC as compared to 5-FU + CDDP [8]. Additional CDDP-based maintenance chemotherapy, as tested in the ACT II trial, did not improve treatment outcome [6]. Although CDDP-based CRT does not improve outcome, it presents an acceptable alternative to MMC because it induces less severe hematologic toxicity.
Only a few studies report the experience of treating anal canal carcinoma in Korean institutions [9-11]. The previous report from our institution showed excellent long-term survival and preservation of anal function with 5-FU + CDDP-based CRT with maintenance chemotherapy. Those patients were treated from 1995 to 2006; the majority of them received conventional radiotherapy and none received the standard 5-FU + MMC regimen [11]. In the present study, all patients received computed tomography (CT) simulation-based three-dimensional conformal radiotherapy (3-DCRT), except for one patient who received intensity modulated radiation therapy (IMRT). Moreover, the majority of patients received 5-FU + MMC-based CRT without NACT or maintenance chemotherapy. We retrospectively analyzed the patients to evaluate treatment outcomes and prognostic factors in anal canal carcinoma.
Materials and Methods
1. Patient eligibility
All patients included in this study were diagnosed with squamous cell carcinoma of the anal canal and treated with curative intent using RT or CRT at Severance Hospital between January 2005 and December 2011. Through medical records, 47 patients were identified. Of these 47 patients, 5 patients received less than 20 Gy, 3 had distant metastasis at initial diagnosis and one was diagnosed with a double primary cancer; these patients were excluded. Among the 5 patients who received less than 20 Gy, 4 patients refused further treatment and 1 patient died of chemotherapy related neutropenic septic shock. Patients who had unilateral external iliac lymph node metastasis and those who underwent transanal excision before curative intent RT or CRT were included.
2. Evaluation
Prior to treatment, patients underwent proctoscopy or sigmoidoscopy, CT or conventional radiography of the chest, CT or magnetic resonance imaging (MRI) of the abdomen and pelvis, and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) with CT. Twenty-eight patients (73.7%) received pelvic MRI, 12 patients (31.6%) received FDG-PET/CT, and 9 patients (23.7%) received both pelvic MRI and FDG-PET/CT for initial staging work-up. Excisional biopsy was performed in two patients who had palpable inguinal lymph nodes, but this was not done routinely. The staging of the tumors was done according to the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for cancer of the anal canal.
3. Treatment
The treatment consisted of curative intent CRT except in patients who were intolerant to chemotherapy. MMC (10 mg/m2) was administered on days 1 and 29 of RT and 5-FU infusion (1,000 mg/m2 per 24 hour) on days 1 to 4 and days 29 to 32 concurrent with radiotherapy. For some patients 5-FU infusion (1,000 mg/m2 per 24 hour) was given on days 1 through 5 and days 29 to 33 plus CDDP (80 mg/m2) given on days 2 and 30. The patients who received 5-FU + CDDP underwent maintenance chemotherapy consisting of the same regimen, repeated every 4 weeks for a maximum 4 cycles.
All patients received external beam radiotherapy with energies ≥6 MV generated by a linear accelerator using a shrinking field technique without a gap. One patient was treated with TomoTherapy Hi·Art II (TomoTherapy Inc., Madison, WI, USA). CT simulation, with the patient in the supine position and with a full bladder, was mandatory. Gross tumor volume included the primary anal tumor and metastatic lymph nodes. Clinical target volume was defined as 2 cm to the primary anal tumor including the mesorectum, as well as bilateral internal and external iliac, bilateral inguinal and presacral nodal regions. The clinical target volume was manually edited to avoid overlap into bone or nontarget muscles.
External beam radiotherapy was performed with either an anteroposterior-posteroanterior or multifield technique. After a dose of 45 Gy in 25 fractions for the whole pelvis, the field was reduced to gross tumor volume plus a 2-cm margin including the mesorectum to deliver an additional boost of 5.4 to 14.4 Gy in fractions of 1.8 Gy; the total dose delivered to metastatic lymph nodes was no more than 54 Gy. In some patients the superior extent of the initial field was reduced at 30.6 to 36 Gy. Negative inguinal nodes received 36 Gy using electrons of 12 to 18 MeV.
4. Follow-up
The first assessments of tumor response were made by clinical and radiologic evaluation (pelvic CT or MRI) 4 weeks and 6 months after the end of RT. Response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Patients were seen weekly during treatment and every 3 to 6 months thereafter. Acute toxicities were recorded according to the National Cancer Institute's Common Toxicity Criteria ver. 3.0 [12] and late toxicities were assessed using long-term radiation sequelae LENT-SOMA (Late effect normal tissues somatic objective management analytic) classification scales [13].
5. Statistical analysis
Locoregional failure was defined as tumor recurrence in the anus or regional lymph nodes and recurrences at other sites were regarded as distant failure. Recurrence-free survival (RFS) was defined from the date of histological diagnosis until recurrence or death by any cause and overall survival (OS) was measured regardless of the cause of death. CFS was based on the date of colostomy or death by any cause, and any patient who underwent a colostomy prior to the CRT was excluded from analysis. Overall treatment time (OTT) was defined as the number of days from the start of RT to the end of treatment.
Data were analyzed using SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). RFS, CFS, OS, and the locoregional control (LRC) rates were estimated using the Kaplan-Meier method. Differences in survival between the groups were tested using a log-rank test. All reported p-values are 2-sided and statistical significance was defined as p < 0.05. Multivariate analyses were performed using Cox proportional hazard models.
Results
1. Patient characteristics and treatment compliance
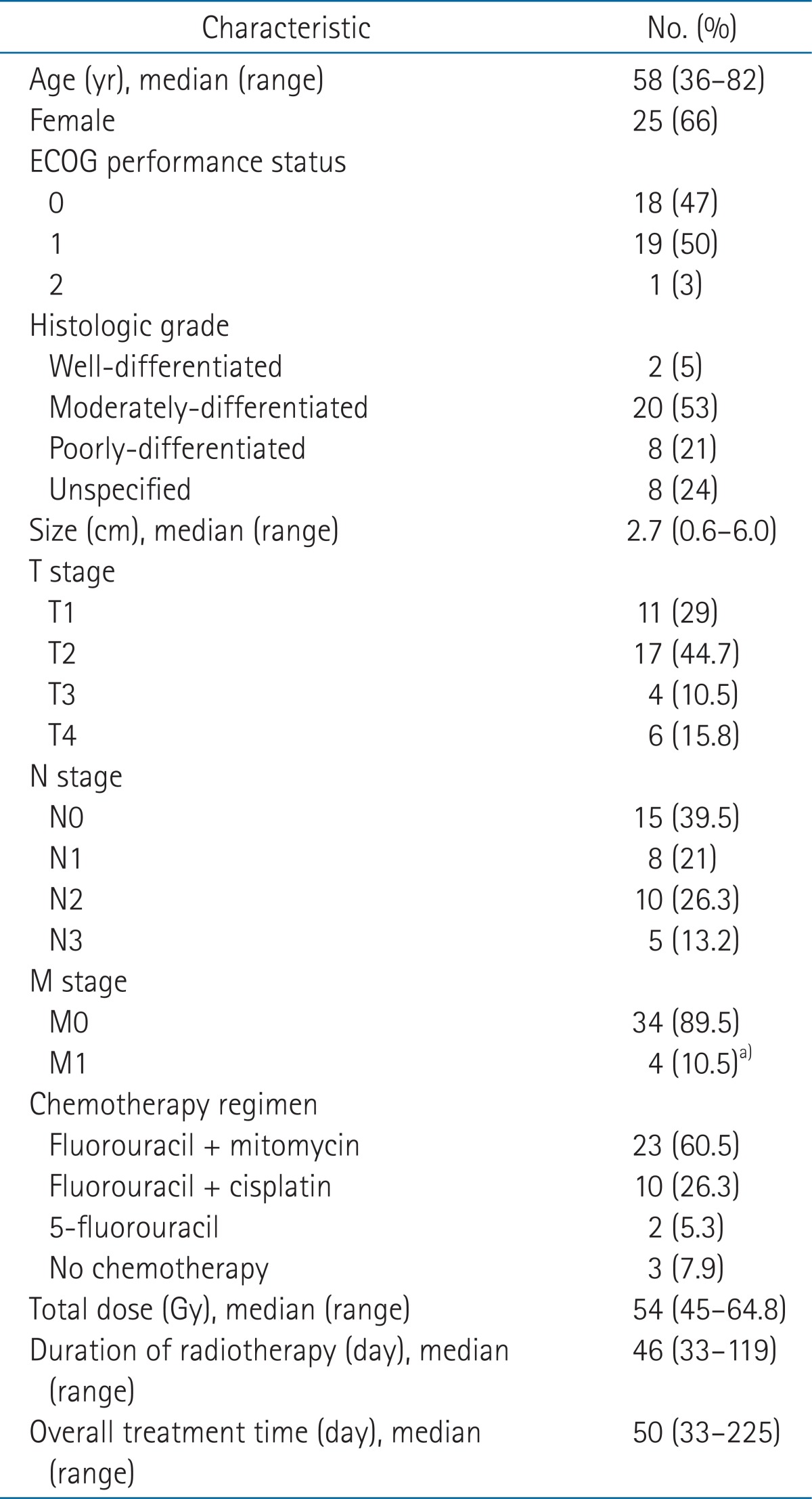
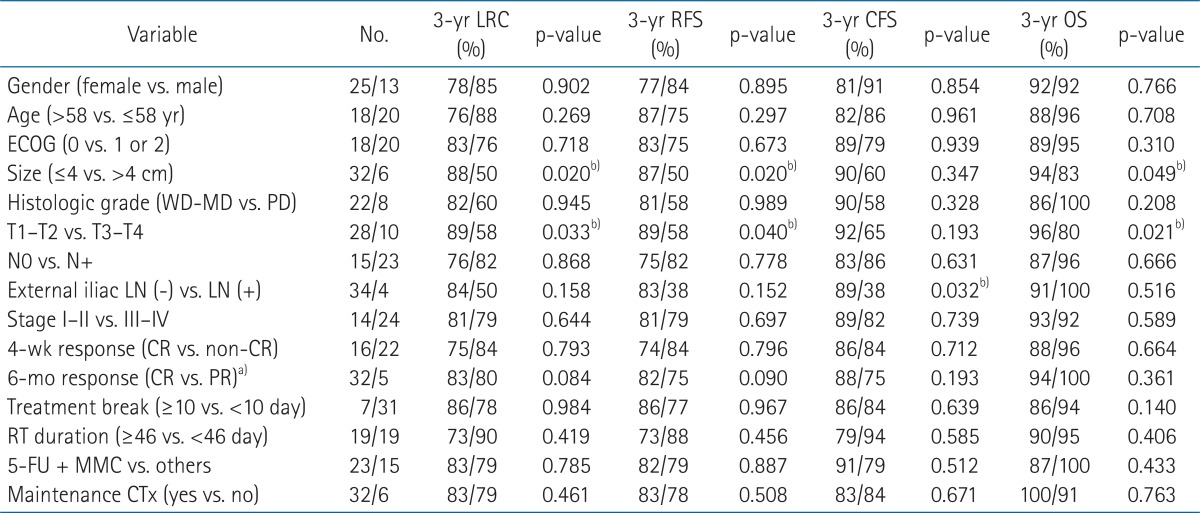
Pretreatment characteristics of 38 patients are summarized in Table 1. The median age was 58 years (range, 36 to 82 years). Twenty-five patients (65.8%) were women; 37 patients (97%) had the Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; tumors were larger than 4 cm in 6 patients (15.8%); 10 patients (26.3%) had T3 or T4 lesions; and 23 patients (60.5%) had radiologically or clinically positive nodes. The stage of disease was I in 5 patients (13.2%), II in 9 patients (23.7%), IIIA in 6 patients (15.8%), IIIB in 14 patients (36.8%), and IV in 4 patients (10.5%).
RT was temporarily interrupted in 13 patients (34.2%) because of toxicity, most commonly dermatitis, but treatment was not prematurely terminated in any patient. The median dose delivered at first treatment break was 41.4 Gy (range, 10.8 to 50.4 Gy). The 9 patients who experienced treatment break due to skin reaction received a median dose of 41.4 Gy (range, 30.6 to 50.4 Gy) at first interruption. The median day of interruption during RT was 10 days (range, 1 to 69 days). One patient with 69 days of interruption underwent curative intent CRT with 50.4 Gy in fractions of 1.8 Gy, but a residual tumor was found at 4-week follow-up. This patient refused surgery and received an additional 10.8 Gy to the primary tumor after a 4-week rest period, which was not related to treatment toxicity. Patients who received more than 54 Gy had more treatment breaks compared to patients who had RT doses of 54 Gy or less, but the difference was not significant (41% vs. 19%; p = 0.167). Chemotherapy was omitted during RT in 2 elderly patients and one patient who had undergone kidney transplantation. Among the 3 patients who underwent RT alone, one received 3 cycles of 5-FU + CDDP after completion of RT. Of the 35 patients who underwent concurrent CRT, 2 received only 5-FU because of advanced age. Six patients (15.8%) received 2 to 4 cycles of maintenance chemotherapy as 5-FU + CDDP. Hematologic toxicities or poor general condition led to chemotherapy dose reductions for 6 patients. The treatment characteristics are summarized in Table 1.
2. Treatment response
The first tumor response evaluation was made by pelvic-CT or MRI at 4 weeks after completion of RT. Sixteen patients (42.1%) had complete response while 21 patients (55.3%) had partial response and one patient had progressive disease. The patient who had progressive disease underwent abdominoperineal resection (APR) 4 weeks after finishing CRT. At 6 months, 16 of the 21 partial responders had achieved complete response. Among the 5 patients with residual tumor shown radiologically at 6 months, 2 underwent biopsy of the primary cancer and were confirmed to have no residual tumor, one experienced local recurrence at 35 months after treatment, and 2 showed no evidence of recurrence.
3. Colostomy
At the time of last follow-up, 5 patients (13.2%) had had a colostomy, 4 of these due to tumor, and one due to underlying Crohn's disease. The patient with Crohn's disease was excluded from analysis. None of the patients had colostomy to address treatment complications. The estimated 3-year CFS was 79.4% (95% confidence interval [CI], 65.5% to 93.3%) (Fig. 1) and the 3-year colostomy rate was 8.1% (95% CI, 0% to 17.1%).
4. Survival and recurrence
At a median follow-up of 44 months (range, 11 to 96 months), 32 patients were alive. Four patients had died from progressive disease, one patient who had had kidney transplantation died from emphysematous pyelonephritis and one died from pneumonia. The latter two were free from active anal canal carcinoma at the time of death. The 3-year OS was 84.5% (95% CI, 71.8% to 97.2%) (Fig. 1). At last follow-up 30 patients were alive without recurrence. The estimated 3-year RFS was 80.0% (95% CI, 66.5% to 93.5%) and median time to first recurrence was 14 months (range, 8 to 39 months) (Fig. 1).
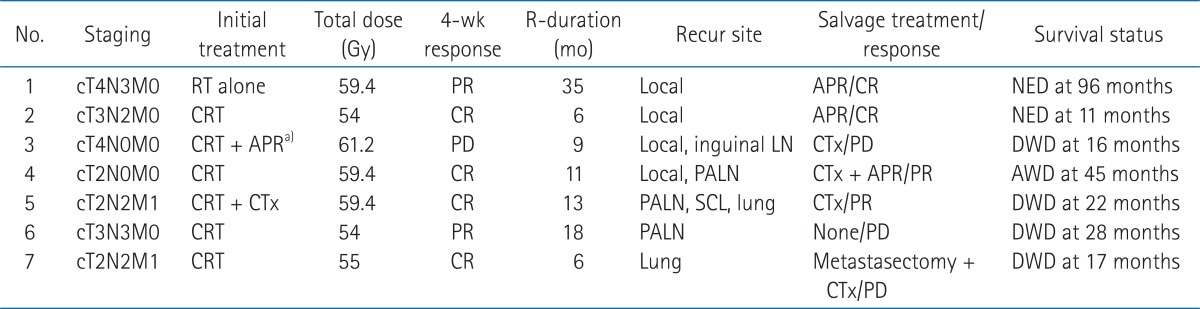
The estimated 3-year LRC rate was 92.1% (95% CI, 83.3% to 100%) and the 3-year distant control rate was 89.0% (95% CI, 78.6% to 99.4%). Seven patients (18.4%) experienced recurrence, including 3 with locoregional failure, 3 with distant failure and one with synchronous locoregional and distant failures. Three patients received APR for local recurrence while one patient received chemotherapy after APR. One patient who had paraaortic lymph node recurrence refused any salvage treatment. Another patient with a single metastatic lung nodule underwent wedge resection but eventually developed pleural metastasis and hematogenous lung metastases. The patient with synchronous local and paraaortic lymph node recurrence had APR and chemotherapy. She is alive without any evidence of local recurrence but with multiple non-regional lymph node and bone metastases. The characteristics of recurrent cases are shown in Table 2.
5. Risk factors
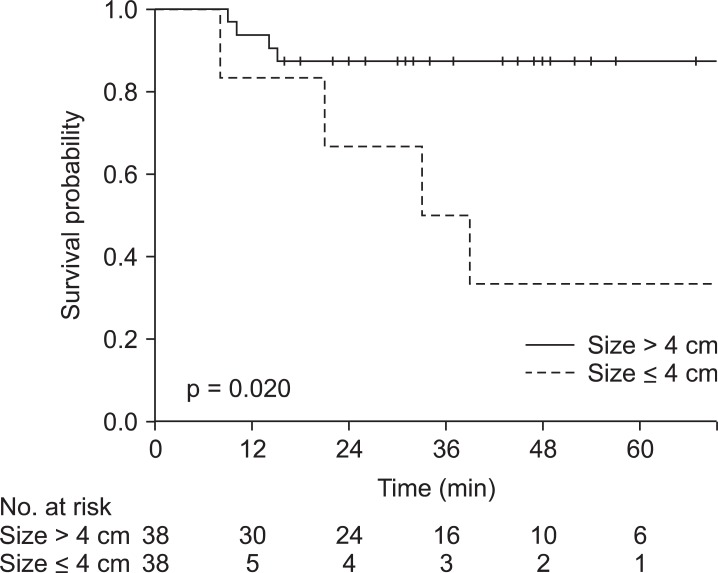
Tumors larger than 4 cm, advanced T stage and the presence of external iliac lymph node metastasis were negatively associated with treatment outcomes (Table 3). The patients with tumors larger than 4 cm had significantly worse 3-year RFS (50% vs. 88%; p = 0.020), 3-year CFS (50% vs. 87%; p = 0.022) and 3-year LRC (94% vs. 83%; p = 0.049) in univariate analysis (Fig. 2). In multivariate analysis tumor size greater than 4 cm was associated with poor RFS (hazard ratio [HR], 6.35; 95% CI, 1.42 to 28.5; p = 0.006) and CFS (HR, 6.25; 95% CI, 1.39 to 28.0; p = 0.017) but not with LRC. Clinical T stage, which includes information about tumor size and tumor extent, was also negatively associated with 3-year RFS (89% vs. 58%; p = 0.033), 3-year CFS (89% vs. 58%; p = 0.040) and 3-year LRC (96% vs. 80%; p = 0.021). Presence of external iliac lymph node metastasis was related to poorer 3-year OS in univariate analysis (89% vs. 38%; p = 0.032) and remained significant in multivariate analysis (HR, 9.32; 95% CI, 1.24 to 70.3; p = 0.030) (Table 4).
Univariate analysis for recurrence-free survival, colostomy-free survival, overall survival and locoregional control (n = 38)
Chemotherapy regimen during CRT did not show significant association with treatment outcome. Patients who received standard CRT with 5-FU + MMC did not differ significantly in RFS, CFS, OS, and LRC from patients who did not receive 5-FU + MMC. Patients who received maintenance chemotherapy did not differ significantly in RFS, CFS, OS, or LRC compared to patients who did not receive maintenance chemotherapy.
The median RT duration was 46 days (range, 33 to 119 days) and median OTT was 50 days (range, 33 to 225 days). Neither RT duration nor OTT was significantly associated with treatment outcome. The RFS, CFS, and OS were not reduced in patients with OTT of 50 days or more compared to patients whose OTT was less than 50 days. The percentage of patients who had OTT ≥ 50 days was significantly higher in the subgroup of patients who underwent maintenance chemotherapy compared to the patients who did not (100% vs. 41%; p = 0.020). RT duration also did not significantly influence RFS, CFS, OS or LRC. Significantly more patients who had RT duration of 46 days or more received RT doses of more than 54 Gy (74% vs. 16%; p < 0.001).
6. Toxicity
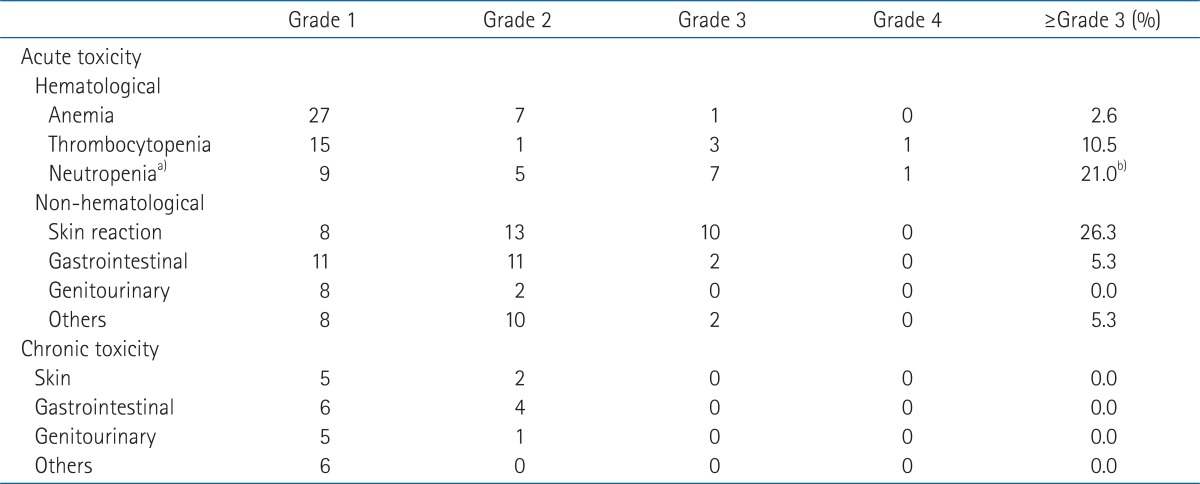
The acute and late toxic effects are summarized in Table 5. The most frequent grade 3 or 4 toxicity was radiation dermatitis. Nine patients had grade 3 or 4 hematologic toxicities and 8 had neutropenia but there were no febrile neutropenia or treatment-related deaths. Among the 23 patients who received 5-FU + MMC, 6 patients (26.1%) developed grade 3 or 4 hematologic toxicities. Of the 15 patients who received chemotherapy other than 5-FU + MMC, 2 patients (15.4%) had grade 3 or 4 hematologic toxicities but the difference between the two groups was not significant (26% vs. 15%; p = 0.273). Other non-hematologic grade 3 or 4 toxicities included diarrhea, anorexia, anal pain and fatigue, each in one patient.
No grade 3 or 4 late toxicities were observed. Grade 2 late toxicities included diarrhea in 2 patients, proctitis in 2, urinary incontinence in one and perineal skin fibrosis in 2.
Discussion and Conclusion
In this cohort of 38 patients who received curative intent CRT for squamous cell carcinoma of the anal canal, the treatment results were comparable to those reported from other studies [3-8,11,14-16]. Although a few studies have reported on treatment outcomes with CRT in anal canal carcinoma using data from individual Korean institutions, the results reported here, gathered from a single, major institution, seems meaningful [9,10]. All patients in our study underwent CT simulation based 3-DCRT and a majority of them received standard 5-FU + MMC-based CRT.
The CR and survival rates were comparable to values previously reported [4,14]. Among the 37 patients whose follow-up imaging evaluations at 6 months were available, 32 patients (86.5%) achieved CR. The previous studies showed CR rates of 80% to 95% at 6 to 12 weeks post-CRT [4,6,14]. In our study the 3-year RFS, CFS, and OS were 80%, 85%, and 79%, respectively. Since 2006, most anal canal carcinoma patients treated in our institution have received 5-FU + MMC concurrently with RT. Prior to 2006, patients received 5-FU + CDDP concurrently with CRT plus maintenance chemotherapy. Patients who received 5-FU + MMC and patients who received other chemotherapy regimens did not differ significantly in RFS, CFS or OS. Efforts to improve treatment outcomes in anal carcinoma have proceeded throughout the past decade. The RTOG 98-11 trial compared the efficacy of the standard 5-FU + MMC regimen and that of 5-FU + CDDP (induction and concurrent). In the long-term follow-up of this trial, the standard 5-FU + MMC regimen showed the more favorable DFS and OS [8]. Preliminary results of the ACT II trial, which compared MMC-based CRT to CDDP-based CRT, showed no difference in response rates or survival between the two groups. Randomization of patients to maintenance chemotherapy or observation also showed no differences in survival [6]. Thus no evidence of benefit in treatment outcome has emerged for additional CDDP-based CRT, NACT or maintenance chemotherapy.
In the present study, tumor size and clinical T stage were significant prognostic factors for RFS, CFS, and LRC. External iliac lymph node involvement was prognostic for OS. Although both clinical T stage and tumor size were statistically significant in univariate analysis, it was reasonable to include only tumor size as a prognostic factor for multivariate analysis since clinical T stage is mostly based on tumor size. In multivariate analysis tumor size larger than 4 cm was independently prognostic for RFS and CFS while external iliac lymph node involvement was independently prognostic for OS. Although a tendency exists in practice to increase the radiation dose according to primary tumor size, patients with larger tumors developed more local recurrences, and this led to colostomy formation. A previous population-based study of 19,199 patients identified male gender, age ≥65 years, advanced T-stage, lymph node involvement, distant metastases, poorly differentiated histology and socioeconomic status as negative prognostic factors for survival [17]. Analysis in the RTOG 98-11 trial revealed tumor size >5 cm (RFS, p = 0.0003; OS, p = 0.0031), clinically positive nodes (RFS, p < 0.0001; OS, p < 0.0001), and male gender (RFS, p = 0.02; OS, p = 0.016) as independently prognostic factors [18]. Recent analysis of the ACT I trial supported lymph node status and gender as independently predictive for LRF and OS as previously reported [19]. No evidence currently supports differential treatment for patients according to prognostic factors. Further understanding of clinical variables as related to tumor biology is essential for further progress.
In our study, increases in neither OTT nor RT duration significantly influenced either locoregional control or survival. Previous retrospective studies show that increases in either RT duration or OTT adversely affect local control and survival [20,21]. Recent analysis of data from the RTOG 87-04 and 98-11 trials combined shows that increase in OTT, but not RT duration, may adversely affect rates of local failure and colostomy [22]. Although the treatment time showed no significant association with treatment outcome in our study, RT duration and OTT seems nevertheless to be important in local control. Our institutional protocol included no treatment break during RT and all patients were treated with 3-DCRT. Despite effort to minimize treatment breaks, 13 patients (34.2%) required interruption for acute treatment-related toxicity. In the interest of reducing acute toxicity, the RTOG 05-29 trial, multi-institutional phase II study assessed the effects of IMRT. Their analysis shows significant reduction in acute grade ≥ 2 hematologic, grade ≥ 3 dermatologic, and gastrointestinal toxicity using IMRT compared to the results in RTOG 98-11 trial [23]. Thus treatment interruptions caused by acute toxicity may be reduced by installation of IMRT.
The limitations of this study include, first, the retrospective design. Secondly, the small sample size and the use of multiple treatment regimens obscured potential relationships between outcomes and treatment factors or risk factors. The patients who were more advanced in stage received higher RT doses which resulted in longer OTT and RT durations. This may have caused the insignificant correlation of the RT factors, such as RT dose or treatment time, with treatment outcomes. Ideally, patients in the same stage group should be analyzed to confirm the influence of RT factors on treatment outcomes, but the small sample size limited further analysis in this study. Another limitation is the relatively short follow-up period (44 months), and this may have resulted in the underestimation of recurrence. However, a 13-year follow-up update of the ACT I trial and another retrospective study, which reported the 30-year experience from a single institution in treating anal canal carcinoma with CRT, showed that 80% to 84% of treatment failures occurred within 2 years [15,24].
Our findings show very favorable results, in terms of RFS, CFS, and LRC, for the use of CRT with curative intent in patients with squamous cell carcinoma of the anal canal. The treatment resulted in no treatment-related deaths or colostomies. Treatment outcomes for patients with large tumors and for those with advanced nodal disease were poor, however. For high-risk patients such as these, new agents and technologies are needed. Radiosensitizing chemotherapeutics and targeted agents currently in development may be useful; and IMRT may assist in the intensification of treatment.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, the Ministry for Health, Welfare & Family Affairs, Korea (A084120).
Notes
No potential conflict of interest relevant to this article was reported.