 |
 |
AbstractPurposePulmonary sarcomatoid carcinoma (PSC) is recognized for its aggressiveness and poor prognosis. The role of radical radiotherapy in PSC remains uncertain due to its scarcity and limited data. In the absence of an effective systemic agent, this study aims to explore the possibility of cure and to investigate potential prognostic factors and treatment outcomes.
Materials and MethodsFrom January 2005 to December 2021, 149 PSC patients were identified. Among 62 patients who received radiotherapy for lung lesions, 25 who underwent palliative radiotherapy and 16 who underwent surgery were excluded.
ResultsThe median patient age was 71 years. The majority were male, and 17 patients (81.0%) were diagnosed at an advanced stage. After radical radiotherapy, distant metastasis (47.6%) was the most common site of failure, while the local recurrence rate was quite low (9.5%). Eventually, five patients (26.3%) demonstrated either a partial response or complete remission, including three complete remissions with durable responses. The median progression-free survival (PFS) and overall survival were 4.6 months and 7.9 months, respectively. Univariate and multivariate analyses revealed that a tumor size >5 cm was associated with a worse prognosis (p = 0.045), while a radiation dose >58 GyEQD2 was significantly associated with better PFS (p = 0.038).
ConclusionThis study demonstrates clinical outcomes after radical radiotherapy in managing PSC, suggesting tumor size and radiation dose could be a predictor of a systemic response. Given the known bad prognosis but complete remission could be achieved in certain subgroups, future research should explore the potential strategies using radical radiotherapy for this challenging patient population.
IntroductionPulmonary sarcomatoid carcinoma (PSC) is a rare and aggressive subtype of non-small cell lung cancer (NSCLC), encompassing five pathologic types: carcinosarcoma, spindle cell carcinoma, pleomorphic carcinoma, giant cell carcinoma, and pulmonary blastoma [1]. With an incidence rate representing less than 1% of all lung cancers, PSC predominantly affects males and smokers [2]. The heterogeneous nature of this NSCLC subtype often leads to its detection at advanced stages due to its aggressive behavior and local invasiveness. Because of the rarity of PSC, there is a paucity of prospective clinical trials aimed at identifying the most effective treatment for this condition. Currently, radical surgery is recommended as the primary curative approach for early-stage patients, but the overall prognosis remains poor, with 5-year overall survival rates ranging between 12.3% and 25.1% [3,4]. Various approaches have been investigated for unresectable PSC but have failed to show significant survival gains. Notably, savolitinib, a selective MET tyrosine-kinase inhibitor, showed a response rate of 49.2% in a recent phase 2 study, which, although promising, is still not high enough [5]. Consequently, there is a demand for local treatment modalities with higher control rates other than surgery.
Radiotherapy (RT) plays a critical role in treating NSCLC, not only in early stages but also in inoperable or advanced stages. A retrospective study analyzing PSC using the Surveillance, Epidemiology, and End Results (SEER) database (n = 1,039) suggested better survival outcomes for patients who received RTвҖ”hazard ratio (HR) = 0.801, p = 0.041. However, due to limitations in database research, not only it is impossible to discern the purpose and timing of RT, but it also does not contain information about RT dose or local responses [3]. Until now, clinical data specifically investigating the therapeutic efficacy and response of PSC to radical RT is currently lacking. In the absence of an effective systemic agent, this study aims to explore the possibility of cure, and to elucidate the prognostic factors, tumor response, and survival outcomes following radical RT.
Materials and Methods1. Patient populationThe study design and protocol were reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2301-043-1393) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB No. 10-2023-34). Patients diagnosed with carcinosarcoma, spindle cell carcinoma, pleomorphic carcinoma, pulmonary blastoma, or those exhibiting focal sarcomatoid features in tissue biopsy results were identified from two institutions between January 2005 and December 2021. Inclusion criteria is patients receiving radical aim of RT for PSC. The exclusion criteria included patients who underwent salvage surgery on the pulmonary mass after radiotherapy and those diagnosed with distant metastasis or another carcinoma at the time of diagnosis. Out of 149 identified PSC patients, 62 had received radiotherapy targeted at lung lesions. Patients who underwent radiotherapy with palliative intent (n = 25) or those who had surgery either before or after radiotherapy (n = 16) were excluded. Consequently, 21 patients who received radical radiotherapy were selected for further analysis. The median follow-up duration was 7.4 months (range, 1.5 to 148.0 months).
2. Diagnostic work up and details of treatmentChest computed tomography (CT) scans were performed to assess the extent of the tumor, while fluorodeoxyglucose-positron emission tomography (FDG-PET) scan was used to evaluate the presence of distant metastases. Tumor pathology was confirmed through tissue biopsy. Pulmonary function tests (PFT) were conducted before treatment initiation. RT doses ranged from 42 Gy to 66 Gy, administered in various fractionation schedules from 4 to 33 fractions. The gross tumor volume (GTV) was delineated for the primary lung tumor and the involved mediastinal lymph nodes. The internal target volume (ITV) was delineated using four-dimensional CT (4D-CT) for patients with tumors in the lower lobe with significant horizontal movement, as well as for those with small tumors for stereotactic ablative radiation therapy. The clinical target volume (CTV) was delineated with an expansion of 3вҖ’7 mm from the GTV, and adjustments were made as needed. The planning target volume was delineated from the CTV/ITV with a 5вҖ’7 mm margin. Concurrent chemoradiotherapy (CCRT) was considered for patients with nodal invasion who had an acceptable performance status. For patients who underwent CCRT, taxane/carboplatin or taxane/cisplatin were mainly applied, and one patient received etoposide/cisplatin due to focal neuroendocrine feature. Programmed death ligand-1 (PD-L1) inhibitor was applied sequentially in selected two PD-L1 positive patients.
3. Follow-up and definition of tumor response and toxicityFollow-up chest CT scans were performed every three months. Tumor response was evaluated by measuring changes in the long axis of the primary lung lesion. A complete response was defined as the disappearance of the lesion on imaging, while a partial response was defined as a decrease of at least 30% in the initial tumor size. The eventual response is defined as the response evaluated at the time of the data analysis. Grade вүҘ3 toxicities, including respiratory, dermatological, and hematological events, were classified according to the Common Terminology Criteria for Adverse Events (version 5.0). Events occurring within 6 months were defined as acute toxicities, while those occurring after 6 months were classified as late toxicities.
4. Statistical analysisProgression-free survival (PFS) was defined as the time interval from the initiation of curative radiotherapy to the time of tumor progression or death from any cause, with censoring applied to patients at their last follow-up. Survival rates were estimated using the Kaplan-Meier method. Overall survival (OS) was defined as the time interval from the initiation of curative RT to death from any cause. Univariate and multivariate analyses were conducted to identify prognostic factors for PFS using the Cox proportional hazards regression model. Backward stepwise selection was adopted for multivariate analyses. The prescribed radiation dose was calculated as the equivalent dose in 2-Gy fractions (GyEQD2, calculated by the Linear-Quadratic model, Оұ/ОІ = 10 Gy). A swimmer plot was adopted to visualize the longitudinal duration of individual overall systemic responses, excluding two patients who were lost to follow-up (n = 19). Each bar represents one patient, with annotation of the initiation of disease progression, partial response, and complete remission along the bar. A bar without any other annotation indicates stable disease. When there was no evidence of systemic progression at the last follow-up, the patient was regarded as having an ongoing response. Durable responses, defined as responses lasting more than 6 months, are annotated separately. A waterfall plot was adopted for visualizing the size change of the primary tumor after treatment, but five patients who were unable to measure the extent of disease due to radiation fibrosis were excluded when evaluating eventual response. All statistical analyses were performed using Stata 16.0 (StataCorp LLC, College Station, TX, USA).
Results1. Patient characteristicsFrom January 2008 to December 2021, a total of 21 patients with pathologically confirmed sarcomatoid carcinoma who received radical RT were included in this study. The majority of these patients were male (n = 18; 85.7%), with a median age of 71 years and generally tolerable performance status. Baseline PFT revealed median values of forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) to be 89% and 92.5%, respectively. More than half of the patients (n = 12; 57.1%) had a history of smoking. As per the American Joint Committee on Cancer 8th edition staging system NSCLC, 17 patients (81.0%) were classified into stage III or IV. Four patients diagnosed with stage IV were treated with a radical aim after 2020, when the concept of oligometastasis is accepted. Among them, two patients had contralateral lung nodule, one patient had suspected malignant pleural effusion without pathological confirmation and one patient had oligometastasis in the brain. Approximately half of the patients (n = 11; 52.4%) were diagnosed with pure sarcomatoid carcinoma, while the rest (n = 10; 47.6%) presented focal sarcomatoid features in their pathology reports. PD-L1 expression was positive in nine patients (42.9%), with the status unknown for 10 patients (47.6%). CCRT was administered to nine patients (42.9%), while 12 patients (57.1%) underwent radiotherapy alone. The median prescribed radiation dose was 60 GyEQD2, with a range from 42 GyEQD2 to 125 GyEQD2.
Among the 19 patients available for response evaluation, five demonstrated a durable response to radical radiotherapy, which response lasted longer than 6 months. In the responder group, defined as those showing complete remission or partial response, all had a primary tumor size of less than 5 cm. The majority of responders had pure PCS (n = 4; 80.0%) as per their pathology reports, and no patient in this group showed negative PD-L1 expression status (range, 40% to 90%). Detailed patient characteristics are summarized in Table 1.
2. Treatment response, survival outcomes, pattern of failure, and prognostic factorsThe median PFS and overall survival for the entire cohort (n = 21) were 4.6 months and 7.9 months, respectively (Fig. 1A). Among the 19 patients with follow-up data, 11 had experienced a good response to radiotherapy in the form of partial response or complete remission in their primary tumors. However, disease progression was observed in six of these patients after confirming their response. Durable response, defined as a response lasting more than 6 months, was observed in five patients, including three with complete tumor remission (Fig. 2). Three patients who achieved complete remissions had PFS of 148.0 months, 19.6 months, and 66.4 months, respectively. Patient #1, a 71-year-old male with stage III disease, had a 3.5-cm tumor and underwent CCRT, receiving a radiation dose of 66 Gy in 33 fractions with docetaxel and cisplatin. Patient #2, a 72-year-old male with stage II disease, presented with a 4.8-cm tumor and underwent radiotherapy alone, receiving 48 Gy in 4 fractions. Patient #3, a 51-year-old female with stage III disease, had a 3.3-cm tumor and underwent CCRT with docetaxel and cyclophosphamide. Ten patients exhibited a response within 1 month of RT, but this response subsided in six of them. The swimmer plot and the waterfall plot of the response is depicted in Figs. 2 and 3, respectively. At 1 month after treatment, follow-up chest CT imaging was available for 19 patients and none of them showed tumor growth after radiotherapy (Fig. 3A). One patient showed no change in the primary tumor while 11 patients (57.9%) displayed a 30% or greater reduction of their tumors. However, two patients experienced progression in distant metastasis without local progression; one patient had progression in both lungs and another in the retrocaval, cardiophrenic lymph node, and adrenal gland. In five patients, post-treatment tumor size became unmeasurable due to RT-induced fibrosis. Among the remaining 14 patients, five (35.7%) eventually showed a response (Fig. 3B).
Distant metastasis (n = 10; 47.6%) was the most common site of failure, often accompanied by regional recurrence (n = 5; 23.8%) and local recurrence (n = 1; 4.8%). Simultaneous regional recurrence and local recurrence occurred in one patient (4.8%), while only regional recurrence was observed in another patient (4.8%). The detailed pattern of failure is presented in Supplementary Table S1.
Univariate analysis of prognostic factors for PFS revealed that tumor size greater than 5 cm was associated with a significantly higher HR of 3.584 (95% confidence interval [CI], 1.030вҖ“12.468; p = 0.045). Smoking history, PD-L1 expression status, distant metastasis, pure PCS, and treatment modalities did not show statistical significance. In the multivariate analysis, administering a higher radiation dose of >58 GyEQD2 was significantly associated with a high PFS (HR = 0.204; 95% CI, 0.046вҖ“0.915; p = 0.038), suggesting a potential benefit of high-dose radiation in treating PCS (Table 2).
3. Adverse events after radical RTOverall, the incidence of acute adverse events was 38.1%, and the incidence of late adverse events was 23.8%. Table 3 presents the adverse events, with a focus on respiratory toxicity. Among the acute adverse events, grade 1-2 respiratory toxicity was reported in eight patients (38.1%). Late respiratory toxicity of grade 1-2 was reported in five patients, and one patient (4.8%) experienced grade 3 respiratory toxicity well managed with supportive care.
Discussion and ConclusionDue to the rarity of PCS, limited research has been conducted, resulting in insufficient evidence for treatment implementation, especially concerning radical RT. In this retrospective study, the entire cohort demonstrated dismal survival, with a median PFS of 4.6 months and a median OS of 7.9 months. However, better PFS was observed in patients with smaller tumors (вүӨ5 cm). Furthermore, multivariate analyses suggested a potential beneficial effect of higher radiotherapy doses >58GyEQD2. Long-term survivors with durable responses were found, providing a clue for achieving cure through radical RT with or without systemic chemotherapy.
PSC is historically known for its aggressive nature and consequent poor outcomes. According to recent SEER database studies, the median OS for the entire PSC patient group was reported as 9 months. However, this increased to 34 months when the disease was localized, with no regional or distant metastases [2,4]. In our cohort, survival outcomes were comparable to those reported in the literature. Notably, stage I-II patients accounted for four patients (19.1%) of our entire cohort (Table 1). Because patients who were suitable candidates for surgery were excluded, resulting in a higher proportion of patients with advanced stage disease. Given that advanced T, N, and M stages are often present at diagnosis in PSC, earlier disease stages (I-II) have been reported in 25.2%вҖ“32.8% of cases in previous studies [1-4,6,7].
To date, the role of radiotherapy in PSC treatment has been consistently reported, but results have been somewhat controversial. Steuer et al. [8], for instance, reported a vague significance of both chemotherapy and radiotherapy compared to surgery in their National Cancer Database-based analysis. However, in a recent retrospective analysis using propensity score matching, patients who underwent RT showed longer OS compared to patients who received only the best supportive care (p < 0.001). The survival benefit was maximized in patients with stage I-III disease, with no survival benefit identified in stage IV patients [9].
In a retrospective study, Ung et al. [6] reported a median OS of 16.4 months for patients who underwent surgery, compared to an extremely dismal prognosis of 3.0 months for those who did not have surgery. Among the patients who did not undergo surgery, 75% were diagnosed with stage IV disease. A total of 28 patients received first-line chemotherapy, and a mere five patients underwent RT in conjunction with chemotherapy [6]. In PCS, resistance to chemotherapy is well documented, with poor response having progression rates reaching up to 85% [6,10]. When compared to the response rate of chemotherapy, a relatively better response was observed following RT. Out of a total of 21 patients, five were responders and 14 were non-responders, excluding two patients who were lost to follow-up. Despite the fact that more than half of the patients had RT alone (57.1%), 10 patients (52.6%) exhibited a decrease of 30% or more in primary tumor size eventually, and 11 patients (57.9%) showed such a decrease 1 month following radical RT (Fig. 3). Notably, given the favorable responses observed in stage IV oligometastatic cases, the use of aggressive RT could potentially improve patient outcomes compared to conventional chemotherapy, depending on the disease presentation and performance status.
Our results demonstrated a significant association between tumor size and treatment response. All the patients who responded to the treatment had a tumor size of вүӨ5 cm. In contrast, 50% of the non-responder had a tumor size of вүӨ5 cm (n = 7). In our univariate analysis, a tumor size >5 cm was associated with poorer PFS (HR = 3.584; 95% CI, 1.030вҖ“12.468; p = 0.045). However, this finding did not reach statistical significance in the multivariate analysis, similar to other variables such as age, gender, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, FEV1, DLCO, disease stage, histology and PD-L1 expression status, which were not significantly associated with treatment response. Distant metastasis demonstrated a trend towards poorer PFS (HR = 2.652; 95% CI, 0.764вҖ“9.202; p = 0.125). The role of distant metastasis as a prognostic factor in PCS has also been reported in previous research [11]. Additionally, in our cohort, one patient received a radiation dose of 42 Gy in 21 fractions due to poor performance status and showed stable disease after RT. The other three patients, who were treated with less than 58 GyEQD2, experienced distant metastases, with or without regional recurrence (Supplementary Table S1). Our multivariate analysis further revealed that receiving a radiation dose >58 GyEQD2 (HR = 0.242; 95% CI, 0.057вҖ“1.030; p = 0.055) was associated with better PFS. Hypofractionation could be considered for some patients who are unable to undergo long-term RT, as observed in our cohort. This information could serve as preliminary evidence suggesting a minimal radiation dose for radical treatment of PSC. Furthermore, in our cohort, the pre-treatment PFT was relatively tolerable, and less than 5% of patients experienced acute or late respiratory toxicity following radical treatment.
The necessity of adjuvant treatment continues to be under investigation, although our study excluded patients who had undergone surgery, the impact of perioperative RT was not analyzed. With a modest benefit in OS with RT over no treatment, Liang et al. [9] reported a trend of a detrimental effect of adjuvant RT after propensity score matching. Furthermore, there is very limited evidence on neoadjuvant radiation. A SEER database-based retrospective study reported that an extremely small portion of patients (n = 76; 1.6%) had undergone neoadjuvant RT, but this was associated with superior OS (p = 0.018) [7]. In our study, less than half (42.9%) of the cohort had undergone concurrent chemotherapy with radiotherapy, which had no clinical significance (HR = 0.988; 95% CI, 0.331вҖ“2.950; p = 0.983). The role of CCRT in locally advanced PSC was also unknown. Conflicting results have also been reported for perioperative chemotherapy in PCS. While researchers at Memorial Sloan Kettering found no difference in survival among recipients of neoadjuvant chemotherapy, a recent meta-analysis showed longer OS with adjuvant chemotherapy (HR = 0.566; 95% CI, 0.439вҖ“0.729; p < 0.001) [12,13]. Investigators at Mayo Clinic suggested potential benefits of perioperative chemotherapy in a univariate analysis [11].
Until now, the appropriate treatment for advanced, inoperable PCS has not been established. The clinical significance of immunotherapy is emerging. Even though prognostic value of PD-L1 expression status have not identified in PSC, previous studies reported the prognostic significance of PD-L1 expression and the presence of metastatic disease in patients with NSCLC [14,15]. Future research should further investigate these factors in the context of PCS and the potential utility of novel treatment approaches, such as immunotherapy, in combination with radiation therapy. Recently, prospective clinical studies of cytotoxicity, targeted therapy, and immunotherapy in PCS have been initiated (NCT03022500, NCT04725448, NCT04888429).
Limitations of our study include the retrospective design and small sample size, as we excluded patients who had palliative RT or who have not undergone RT. Despite these limitations, our study provides real-world clinical data on the response from radical RT, suggesting the feasibility of active RT for PSC including oligometastasis. Further prospective efforts are needed to confirm these results and to establish the optimal treatment approach for patients with PSC.
In conclusion, our study supports the feasibility of radical RT in managing PCS, demonstrating the potential significance of a high biologically effective radiation dose >58 GyEQD2. In the absence of an effective systemic agent, durable response was observed in 26.3% of the patients and long-term survivors were found. However, given the limited sample size, larger prospective studies are warranted to validate these findings and to identify optimal treatment strategies for this challenging patient population.
NotesStatement of Ethics The Institutional Review Board of Seoul National University Hospital (No. H-2301-043-1393) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (No. 10-2023-34) approved this study and waived the requirement for patient informed consent. Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2023.00437.
Fig.В 1.(A) Kaplan-Meier analysis of progression-free survival (PFS) and overall survival (OS) following radical (chemo)radiotherapy for the total cohort. (B) Kaplan-Meier analysis of PFS, stratified by the delivered radiation dose. 
Fig.В 2.Swimmer's plot of systemic responses (n = 19). Each bar represents one subject in the study. A bar without any symbol indicates stable disease. A durable responder is a subject who has a confirmed response for at least 183 days (6 months). 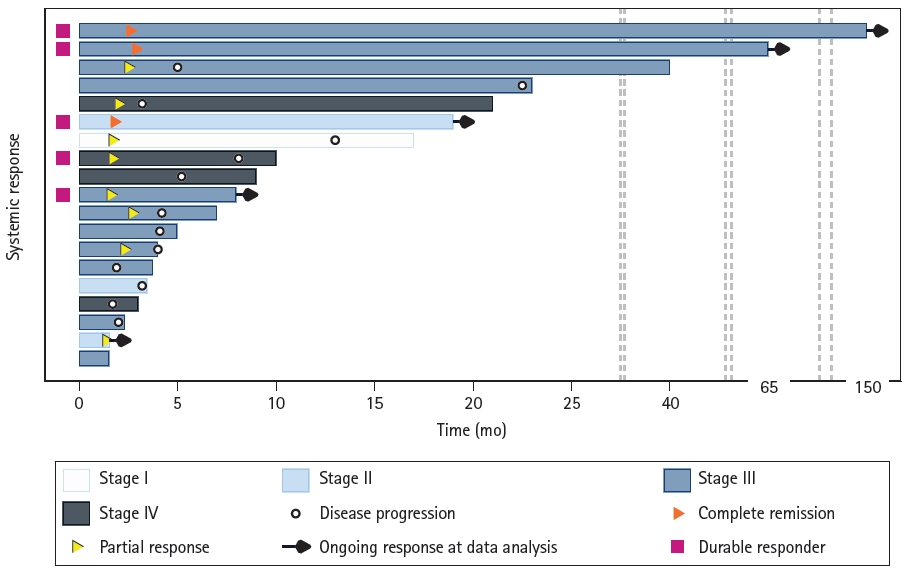
Fig.В 3.Waterfall plot of primary tumor response to (chemo)radiotherapy, based on the time of response evaluation: (A) response after 1 month (n = 19) and (B) eventual response (n = 14). One patient showed no difference in tumor size after 1 month, and five patients were unable to measure disease extent at the time of the data analysis. The Y-axis depicts the percentage of tumor reduction from the baseline. The dotted line signifies a 30% reduction in tumor size, which defines partial response according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria. 
TableВ 1.Patient characteristics
TableВ 2.Univariate and multivariate analysis of prognostic factor for progression-free survival
References1. Chen M, Yang Q, Xu Z, et al. Survival analysis and prediction model for pulmonary sarcomatoid carcinoma based on SEER database. Front Oncol 2021;11:630885.
2. Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012;152:397вҖ“402.
3. Gang J, Yan Q, Xiang S, Zheng L, Zhao L. Clinicopathological characteristics and prognostic factors of pulmonary sarcomatoid carcinoma: a large population analysis. Ann Transl Med 2021;9:121.
4. Yin J, Yang Y, Ma K, et al. Clinicopathological characteristics and prognosis of pulmonary pleomorphic carcinoma: a population-based retrospective study using SEER data. J Thorac Dis 2018;10:4262вҖ“73.
5. Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med 2021;9:1154вҖ“64.
6. Ung M, Rouquette I, Filleron T, et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer 2016;17:391вҖ“7.
7. Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg 2018;53:828вҖ“34.
8. Steuer CE, Behera M, Liu Y, et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin Lung Cancer 2017;18:286вҖ“92.
9. Liang X, Cheng Y, Yuan Z, et al. Clinical, pathological and treatment factors associated with the survival of patients with pulmonary sarcomatoid carcinoma. Oncol Lett 2020;19:4031вҖ“9.
10. Bae HM, Min HS, Lee SH, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007;58:112вҖ“5.
11. Maneenil K, Xue Z, Liu M, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer 2018;19:e323вҖ“33.
12. Chaft JE, Sima CS, Ginsberg MS, et al. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol 2012;7:1400вҖ“5.
13. Zombori-Toth N, Kiss S, Ostarijas E, Alizadeh H, Zombori T. Adjuvant chemotherapy could improve the survival of pulmonary sarcomatoid carcinoma: a systematic review and meta-analysis. Surg Oncol 2022;44:101824.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |