 |
 |
AbstractPurposeThe study evaluates accelerated hypofractionated radiotherapy (AHRT) compared to conventional fractionation radiotherapy (CFRT) in patients with locally advanced head and neck cancer (LAHNC) receiving definitive chemoradiation therapy.
Materials and MethodsThe study includes a retrospective cohort analysis of 120 patients. CFRT arm (n = 65) received 2 Gy per fraction to a dose of 70 Gy over 7 weeks in a three-volume approach, whereas the AHRT arm (n = 55) received 2.2 Gy per fraction to a dose of 66 Gy in 6 weeks with a two-volume approach. The primary outcome was overall survival (OS).
ResultsWith a median follow-up of 18.9 months, 23 patients died in the AHRT arm, and 45 deaths in the CFRT arm. The median OS was 23.4 and 37.63 months in the CFRT and AHRT arms, respectively (hazard ratio [HR] = 0.709; 95% confidence interval [CI], 0.425–1.18; p = 0.189). The median time to loco-regional control was 33.3 months in the CFRT arm and was not reached in the patient group receiving AHRT (HR = 0.558; 95% CI, 0.30–1.03; p = 0.065). The median progression-free survival was 15.9 months in the CFRT arm and 26.9 months in the AFRT arm (HR = 0.801; 95% CI, 0.49–1.28; p = 0.357). Out of 11 acute toxic deaths, eight were in the CFRT arm.
IntroductionHead-and-neck carcinoma (HNC) is a significant contributor to the cancer burden in India, accounting for 21.3% of cases [1]. A majority of patients (over 65%) present with locally advanced disease, for which concurrent chemoradiation (CRT) is the mainstay of treatment, providing a 5-year overall survival (OS) benefit of 6.5% compared to radiation therapy (RT) alone [2]. The standard practice of conventional fractionated RT (CFRT) involves 70 Gy delivered in 35 fractions using a three-volume approach over 7 weeks. However, our experience has shown poor locoregional control (LRC) and OS with this regimen, as well as significant grade 3–5 acute toxicities [3,4].
To improve outcomes, an alternate regimen of accelerated hypofractionated RT (AHRT) delivering 66 Gy in 30 fractions over 6 weeks has been adapted, resulting in increased LRC and OS rates [5,6]. Using the AHRT regimen, the 2-yeare OS was 95.5% and the 2-year LRC rates were 91% without the addition of concurrent chemotherapy in early oropharyngeal carcinoma [7]. For advanced carcinomas of the oropharynx, the 3-year LRC rate was 92% and OS was 83% [8]. The reduction in the overall treatment time (OTT) by 1 week accelerated RT improves the LRC and disease-free survival [9,10]. At our institute, we switched from CFRT to AHRT with the hypothesis that it would deliver a biologically equivalent dose while reducing OTT and improving outcomes. This retrospective cohort study compares the two radiotherapy regimens (CFRT vs. AHRT) and includes measures to reduce acute toxicity like optimizing the dose to dysphagia-aspiration related-structures, weekly concurrent chemotherapy, and surveillance of sepsis.
Materials and Methods1. Study design and settingThis retrospective cohort study was conducted at the Department of Radiation Oncology, St John’s Medical College and Hospital, Bengaluru, following Institute Ethical Clearance. The study included patients with locally advanced (stage III, IVA and IVB) head-and-neck cancer (LAHNC) who had been treated with definitive CRT between January 2013 and December 2021. Patients who had previously undergone head-and-neck irradiation were excluded. Patients were evaluated in a multidisciplinary tumour board, and disease staging was done according to the American Joint Committee on Cancer (AJCC) 8th edition [11]. The cancer staging was reconstructed according to AJCC 8th edition treated prior to 2017. Pre-treatment baseline assessments included a complete blood count, renal function test, liver function test, creatinine clearance, and computed tomography (CT) scan. Data was collected from radiotherapy review charts and follow-up records, and included patient characteristics, disease characteristics, RT details, chemotherapy details, outcome details, and toxicities.
2. TreatmentAll patients received definitive CRT with intensity-modulated radiotherapy (IMRT) technique and 6-MV photons after immobilization with thermoplastic masks. All patients underwent a contrast-enhanced CT simulation with 2.5-mm slice thickness from vertex to the carina. The segmentation was done on the MONACO workstation. Patient in CFRT arm, gross tumor volume (GTV), high-risk clinical target volume (CTV1), intermediate-risk CTV (CTV2), and low-risk CTV (CTV3) were defined. High-risk planning targe volume (PTV1), intermediate-risk PTV (PTV2), and low-risk PTV (PTV3) were generated with an isotropic expansion of 3–5 mm from CTV1, CTV2, and CTV3, respectively. The target volumes, i.e., PTV1, PTV2, and PTV3, were irradiated to a total dose of 70, 63, and 56 Gy in conventional fractionation, respectively. In the AFRT arm, only two volumes were defined: CTV1 and CTV3. The CTV2 was removed. An isotropic expansion of 3–5 mm from CTV is given to generate respective PTV. PTV1 and PTV2 received a dose of 66 and 54 Gy, respectively in AHRT arm. All organ-at-risk (OAR) structures were contoured, such as parotids, submandibular glands (SMGs), pharyngeal constrictors (PC), larynx, and cervical esophagus (CE). The PC was contoured from the pterygoid plates to the inferior border of the cricoid cartilage. The CE was contoured from the lower end of the PC to the lower edge of the C7 vertebral body. The dose constraints used were: spinal cord (Dmax <44 Gy), brainstem (Dmax <54 Gy), parotid (Dmean <26 Gy), SMG (Dmean <35 Gy), PC (Dmean <45 Gy), larynx (Dmean <45 Gy), and CE (Dmean <45 Gy). A 7-field IMRT plan with 6-MV photons was generated using the MONACO treatment planning system [12]. Patients in the AHRT arm received 66 Gy in 30 fractions at 2.2 Gy per fraction to PTV1 and 54 Gy in 30 fractions to PTV2, 5 fractions a week over 6 weeks. Concurrent chemotherapy in the CFRT arm consisted of either weekly cisplatin chemotherapy at 40 mg/m2 or 3 weekly cisplatin chemotherapy at 100 mg/m2, as decided by the treating medical oncologist. All patients in the AHRT arm received concurrent weekly cisplatin chemotherapy at 40 mg/m2 weekly. Chemotherapy was not given after completion of RT. Hydration, anti-emetics, and dose modifications were done according to the National Comprehensive Cancer Network guidelines. Active surveillance of sepsis was done for patients since June 2018.
3. Sepsis surveillanceThe initial experience with 70 Gy in 35 fractions using a three-volume approach showed a high incidence of acute toxicities, leading to treatment discontinuation or death. This was attributed to a toxicity syndrome called the "mucositis-dysphagia-aspiration-sepsis" complex [13]. To address this, a more stringent review process was implemented during the course of concurrent CRT to monitor for sepsis. This involved meticulous monitoring of symptoms, vitals, and blood counts, with steps taken to prevent infection and sepsis. If it was indicative of impending development of infection and sepsis, steps were taken to prevent the same and halt progression. First chemotherapy, hypothesised to aggravate grade 3–4 toxicities was withheld. Conservative management in the form of hydration, antibiotics, and granulocyte colony stimulating factors were tried. The last resort was to withhold RT.
4. Follow-upDuring CRT, all patients were reviewed at least twice a week. After completion of scheduled treatment, patients were followed up weekly until acute reactions subsided, then monthly until 3 months, 3 monthly until 2 years, and then yearly. The response to treatment was evaluated after 8–12 weeks of completing RT with clinical, endoscopy, and/or imaging.
5. Outcome measuresThe primary outcome was median OS. The secondary outcomes were LRC, progression-free survival (PFS), determination of factors affecting the OS, treatment compliance, and the incidence of acute toxicity. OS is defined from the time of diagnosis to death due to any cause. LRC was calculated from the time of diagnosis to locoregional disease recurrence or death due to any cause. PFS was calculated from the time of diagnosis to any disease event, i.e., recurrence (locoregional or distal), second primary or death due to any cause. Toxicity grading was done with the Common Terminology Criteria for Adverse Events v4.03. For assessing compliance, median radiation dose received, duration of concurrent CRT schedule, the number of patients receiving planned radiation or chemotherapy, and the adequate cumulative dose of cisplatin were compared between the two groups.
6. Statistical analysisThe study population size was based on consecutive convenience sampling. The data were analysed using STATA software version 16 (StataCorp LLC, College Station, TX, USA). All categorical data were presented using frequency and percentages, and all continuous data using mean and standard deviation or median and inter-quartile range (IQR) based on the distribution. OS, PFS, and locoregional progression-free survival (LRPFS) were analysed with Kaplan-Meier survival methods and compared using log-rank tests. Univariate and multivariate analyses were performed using Cox proportional hazard regression analysis. The difference in acute toxicities between the two arms was compared with chi-square test. A p-value was considered significant at a 5% level of significance for all comparisons.
Results1. Baseline characteristicsA total of 120 patients with LAHNC were treated from January 2013 to December 2021. Of these, 65 patients received CFRT between January 2013 and May 2018, and 55 patients received AHRT from June 2018 onwards. Table 1 shows the baseline characteristics of the patients. The median age of the cohort was 59 years, and 78.3% of the patients were male. Most patients (95%) had an European Cooperative Oncology Group (ECOG) performance status of 0/1, and 79.1% of the patients had a history of tobacco usage. The oropharynx was the most commonly involved subsite (30.8%), followed by the oral cavity (22.5%). Around 85% of the patients had a T3/T4 primary lesion, 76.7% had lymph nodes involved, and 65.8% had Stage IVA/B disease. In the CFRT arm, around 63% of the patients received weekly concurrent chemotherapy, while 37% received 3-weekly chemotherapy. All patients receiving AHRT received weekly chemotherapy.
2. Treatment complianceThe median duration of RT completion was 46 days (6.6 weeks) and 40 days (5.7 weeks) in the CFRT and AHRT arms, respectively, and the median radiation dose received was 66 Gy for both groups. Only 84.2% of the patients received the planned dose, with acute toxicities being the main reason for not completing RT. About 40% of the patients had unplanned breaks of more than 2 days during the RT course. Twenty-six patients in CFRT arm received 3-weekly cisplatin chemotherapy. Rest of the patients in CFRT arm and all in AHRT arm received weekly cisplatin chemotherapy. Only 28.3% of the patients received the planned chemotherapy cycles. Table 2 provides additional details regarding treatment compliance.
3. OutcomeThe median follow-up period for the entire cohort was 18.9 months (IQR, 8.4 to 41.7 months); with a median follow-up of 23 and 16.5 months for CFRT and AHRT arms, respectively. Complete response was achieved in 56.9% (n = 37) of CFRT and 56.4% (n = 31) of AHRT arm patients. Partial response was seen in 24.6% (n = 16) and 30.9% (n = 17) of CFRT and AHRT arms, respectively. Response status was unknown for 12 (18.5%) and 2 (12.7%) patients receiving CFRT and AHRT, respectively. A total of 42 patients had locoregional progression, with 27 in the CFRT arm and 15 in the AHRT arm. The median time to locoregional progression was 33.3 months in the CFRT arm and not reached in AHRT group (HR = 0.558; 95 % CI, 0.300–1.038; p = 0.065) (Fig. 1). The number of patients with locoregional relapse (LRR), distal metastasis, and both (LRR and distal) in the CFRT and AHRT arms were 21 and 9, 4 and 4, and 2 and 2, respectively (Supplementary Table S1). The median PFS was 15.9 months in the CFRT arm and 26.9 months in the AHRT arm (HR = 0.801; 95 % CI, 0.499–1.285; p = 0.357) (Fig. 2).
In the cohort, there were a total of 68 deaths, with 45 in the CFRT arm and 23 in the AHRT arm. The median OS was 23.4 months in the CFRT arm, while it was 37.6 months in the AHRT arm (HR = 0.709; 95% CI, 0.425–1.184; p = 0.189) (Fig. 3). Of the 45 deaths in the CFRT arm, 29 were caused by the disease, eight were due to acute toxicities related to treatment, and eight were caused by other reasons (cardiac, natural causes, one patient committed suicide). In the AHRT arm, 15 of the 23 deaths were caused by disease progression, three were due to acute toxicities, one death occurred due to the development of a second primary, and four were due to cardiac causes. The HR of death was 0.709 (95% CI, 0.425–1.184; p = 0.189) in patients receiving AHRT arm compared with CFRT arm. On Cox univariate analysis, age over 60 years and AJCC stage group IVA/B had a statistically significant negative impact on OS. On Cox multivariate regression analysis, there was no significant difference in OS between the two arms based on gender, ECOG, Charlson Comorbidity Index, T stage, N stage, and AJCC stage group. Only age >60 years maintained its significant negative impact (Table 3).
4. ToxicitiesGrade 3–4 acute toxicities developed in 49 (52.1%) patients in the CFRT arm as compared to 45 (47.9%) patients in the AHRT arm. The cumulative incidence of acute grade 3–5 dermatitis, mucositis, pain, dysphagia, and aspiration in the CFRT and AHRT arms were 3.1% and 14.5%, 36.9% and 56.4%, 32.3% and 70.9%, 58.5% and 29.1%, 15.4% and 3.6%, respectively (Table 4). There was a total of 11 deaths due to acute toxicity, with eight in the CFRT arm and the remaining three in the AHRT arm.
Discussion and ConclusionThe results of the study suggest a potential benefit in OS, PFS, and LRPFS with AHRT compared to CFRT, although the difference was not statistically significant. Both treatment arms had similar rates of complete response, and the median radiation dose was 66 Gy. However, less than one-third of patients received the planned cycles of chemotherapy due to acute toxicities, which were the main reason for treatment interruption. About 50% of all patients developed grade 3–4 toxicities, and 11 deaths occurred due to acute toxicities. Nevertheless, treatment-related acute toxic deaths were reduced in the AHRT arm, although not statistically significant.
AHRT appears to be a promising treatment option for early oropharyngeal carcinomas, with a 2-year locoregional failure rate of 9% and a 2-year OS of 95.5% [7]. In advanced oropharyngeal carcinomas, AHRT resulted in a 3-year LRC rate of 92% and an OS of 83% [8]. However, when compared with another regimen of 69.96 Gy/33 fractions, AHRT showed lower LRC rates of 72.6%. Nonetheless, there was no difference in the OS and disease-free survival (DFS) [14]. Another study comparing AHRT to CFRT reported a non-significant benefit of AHRT over CFRT in terms of 2-year DFS (62.1% vs. 56.3%; p = 0.640) and 2-year OS (53% vs. 44.5%; p = 0.510), with a 2-year LRF rate of 27.2% versus 33.8% in CFRT and AHRT arms, respectively [15]. These results are consistent with the present study and the original hypothesis that reducing the overall treatment time by 1 week leads to better tumour control, which may be reflected in better OS.
A two-volume approach avoiding intermediate risk volume has the potential to reduce the dose received by OARs and hence reduce the toxicities associated with it. The dosimetry comparison of two- and three-volume approach in head-and-neck treatment plans showed similar intermediate-risk CTV coverage and similar V95% in both two- and three-volume plans for the same case (although two volumes delivered slightly lower dose). The two-volume approach however was more likely to have cold spots at the periphery of the intermediate-risk region [16]. In another study considering human papillomavirus positive oropharyngeal carcinoma who received definitive radiotherapy, a retrospective delineation of intermediate-risk PTV was done after documented LRR. No potential patients were found whose recurrences could have been prevented by giving an intermediate-risk dose. So, emitting the same and following a two-volume approach may be acceptable [17].
Sepsis is a life-threatening complication that can occur in cancer patients undergoing chemotherapy and RT. The risk of sepsis is particularly high in patients with HNC due to the immunosuppressive effects of treatment and the potential for treatment-related mucositis and infections. To reduce the incidence and severity of sepsis in HNC patients undergoing CRT, surveillance protocols have been proposed. These protocols involve close monitoring of patients for signs and symptoms of infection, such as fever, chills, and difficulty swallowing, and prompt initiation of appropriate antibiotic therapy when necessary. A literature review and consensus statement by Mirabile et al. [18] suggested that sepsis surveillance during CRT for HNC may result in a reduction in treatment-related acute toxic deaths. In the present study, active sepsis surveillance during CRT for HNC patients was found to be beneficial in reducing treatment-related acute toxic deaths in the AHRT arm, although not statistically significant. This was reflected in a higher percentage of patients completing RT as planned, although with a higher number of breaks during RT in the AHRT arm due to acute toxicities. The cumulative incidence of acute dermatitis, mucositis, pain, and dysphagia was more in AHRT arm, but incidence of aspiration and acute toxic deaths were increased in CFRT arm. This may be due to retrospective nature of the study, and better monitoring and toxicity recoding in AHRT arm done for sepsis surveillance.
Despite the promising findings of this study, there are few limitations that must be considered. First, the study is retrospective in nature, which means that it is subject to the biases and limitations inherent in such studies. Additionally, the two treatment arms were tested at different time points, with median follow-up of AHRT arm lesser than the CFRT arm. This could have introduced confounding variables that were not accounted for in the analysis. Additionally, the study had a relatively small sample size, with 120 patients included in the analysis. The decrease in toxic deaths by surveillance of sepsis is still a hypothesis and has to proven in a prospective study, after clearly defining the surveillance parameters. Also, 3-weekly concurrent cisplatin was received by 40% (n = 26) patients in CFRT arm and none in AFRT arm. This might be one of the reasons for more toxic deaths in CFRT arm. Another limitation is the subjective grading of acute toxicities, which could have introduced variability in the data. Furthermore, the study had a relatively short follow-up period, which may not have been long enough to fully evaluate the benefits of AHRT over CFRT. Longer follow-up is needed to assess the true benefits of AHRT in terms of OS, PFS, and LRC. Moreover, the study population was limited to patients with LAHNC stage III or IV, with unresectable disease and history of tobacco usage. This limits the generalizability of the findings to other patient populations. Furthermore, the study did not include patients with comorbidities, which are common in the older adult population. Finally, the study did not evaluate the impact of AHRT on patient quality of life, which is an important consideration in cancer treatment.
The strength of the study lies in its real-world setting, where patients encountered in the clinical setting are similar to those in the study. Patients with locally advanced head and neck cancer, stage III or IV, with unresectable disease and history of tobacco usage were included in the study, which is representative of the patient population that typically presents to radiation oncology clinics in India. Another strength of the study is the comparison of AHRT and CFRT, which are both commonly used treatment approaches, and the results provide valuable insight into the potential benefits and limitations of these approaches. The study also used rigorous statistical analysis to compare the outcomes between the two treatment arms, which add to the strength of the study. The use of a two-volume approach for RT and sepsis surveillance during CRT course is another strength of the study, as it resulted in reduced treatment-related acute toxic deaths in the AHRT arm.
Overall, the study provides valuable information on the potential benefits and limitations of AHRT and CFRT in the treatment of locally advanced HNC. The study's real-world setting and rigorous statistical analysis add to the strength of the study, while the use of a two-volume approach for RT and sepsis surveillance during CRT course reduces treatment-related acute toxic deaths. The study provides valuable insights into the potential benefits and limitations of different treatment approaches for HNC and adds to the body of literature on the use of AHRT in this patient population. The findings of the study could be useful for radiation oncologists and clinicians involved in the treatment of LAHNC, providing an insight into the efficacy and safety of AHRT, and the potential benefits of the two-volume approach for RT and sepsis surveillance during CRT course. Further studies with larger and more diverse patient populations are needed to confirm the findings and address the limitations of this study.
In conclusion, the present study suggests a potential benefit of AHRT compared to CFRT in terms of LRC in patients with LAHNC receiving CRT. However, the difference was not statistically significant. The PFS and OS were similar in both the arms. AHRT appears to be a promising treatment option for patients with LAHNC fit for CRT. A longer follow-up of patients receiving AHRT is required to assess the benefit.
NotesStatement of Ethics The study was approved by the Institutional Ethics Committee of St. Johns Medical College with IEC reference No. 198/2022. Being a retrospective analysis, permission for waiver of consent was obtained. Acknowledgements The authors thank all the staff of department of Radiation Oncology, Medical Oncology, Surgical oncology, Pain & Palliative, and ENT at St John’s Medical College and Hospital. Author Contributions Conceptualization, Muzumder S, Tripathy A. Investigation and methodology, Tripathy A, Muzumder S, Srikantia N, Udayashankar AH. Writing of the original draft, Tripathy A, Muzumder S. Writing of the review and editing, Srikantia N, Vashishta GD, Udayashankar AH, John Sebastian MG. Validation, Tripathy A, Muzumder S, Srikantia N, Udayashankar AH. Formal analysis, Raj JM, Tripathy A. Data curation, Tripathy A, Babu A, Muzumder S, John Sebastian MG, Udayashankar AH, Vashishta GD. Visualization, Tripathy A, Muzumder S, John Sebastian MG. Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2023.00248.
Fig. 1.Kaplan-Meier estimates of locoregional control. CFRT, conventional fractionated radiation therapy; AHRT, accelerated hypofractionated radiation therapy; HR, hazard ratio; CI, confidence interval. 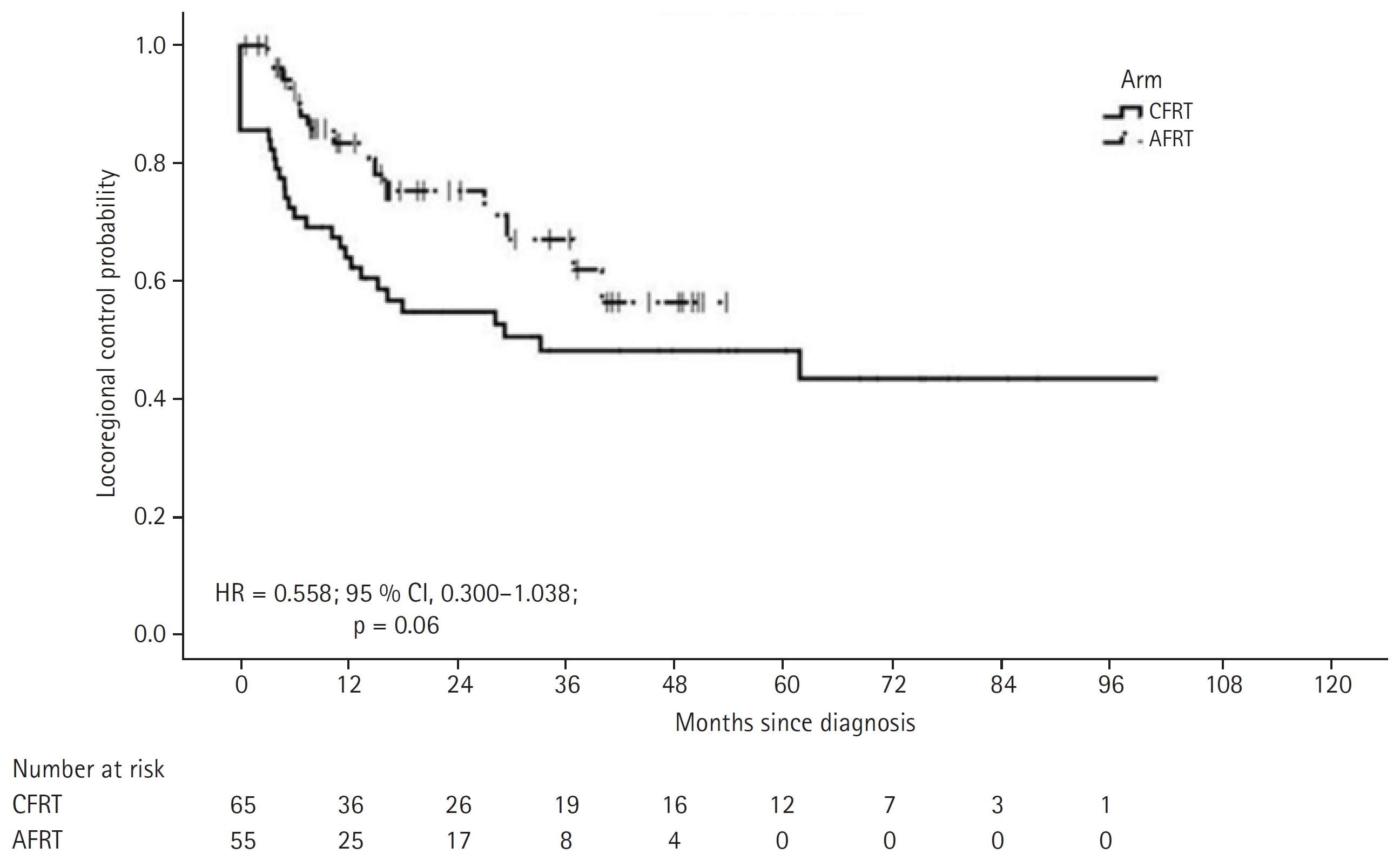
Fig. 2.Kaplan-Meier estimates of progression-free survival. CFRT, conventional fractionated radiation therapy; AHRT, accelerated hypofractionated radiation therapy; HR, hazard ratio; CI, confidence interval. 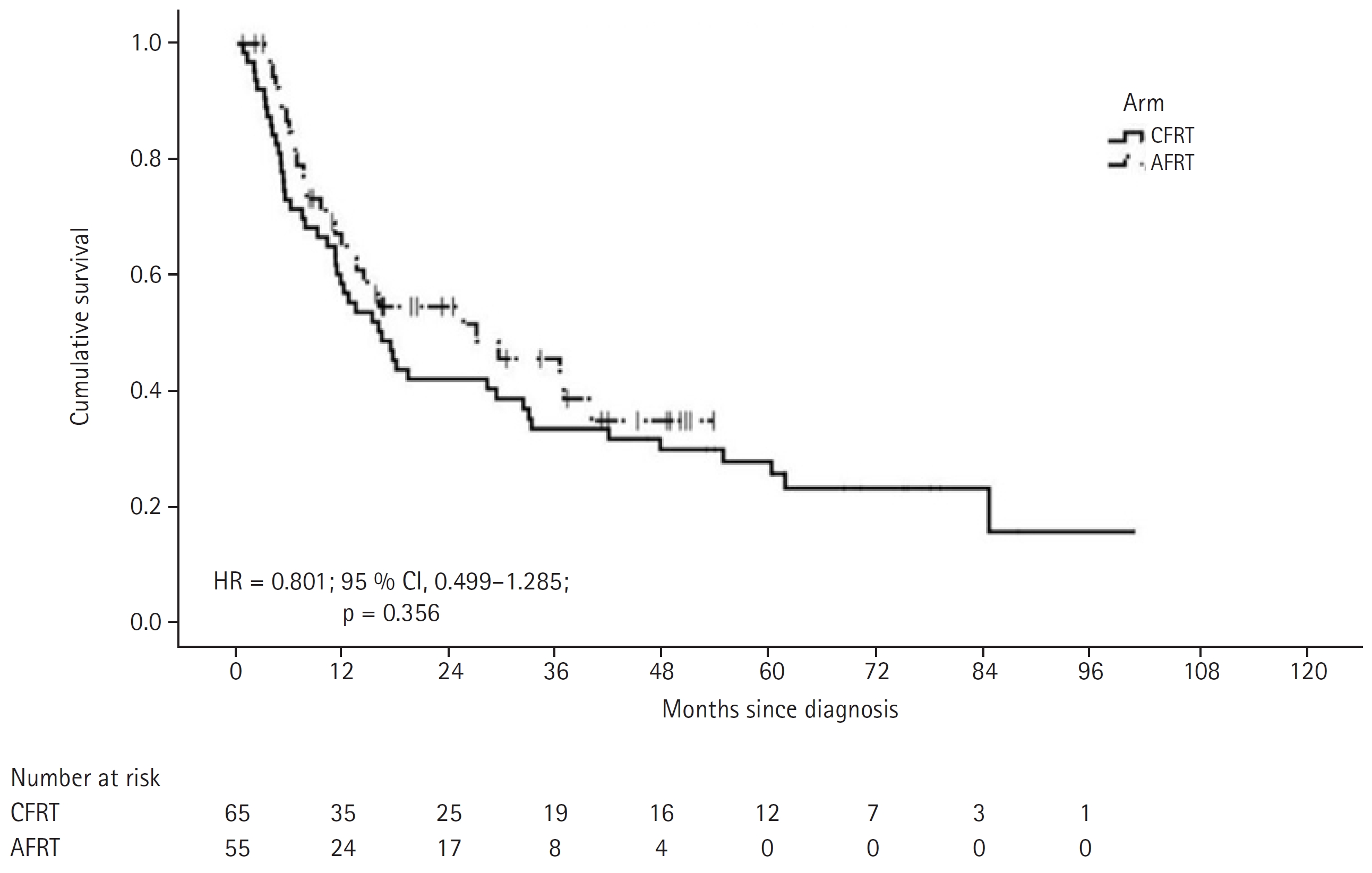
Fig. 3.Kaplan-Meier estimates of overall survival. CFRT, conventional fractionated radiation therapy; AHRT, accelerated hypofractionated radiation therapy; HR, hazard ratio; CI, confidence interval. 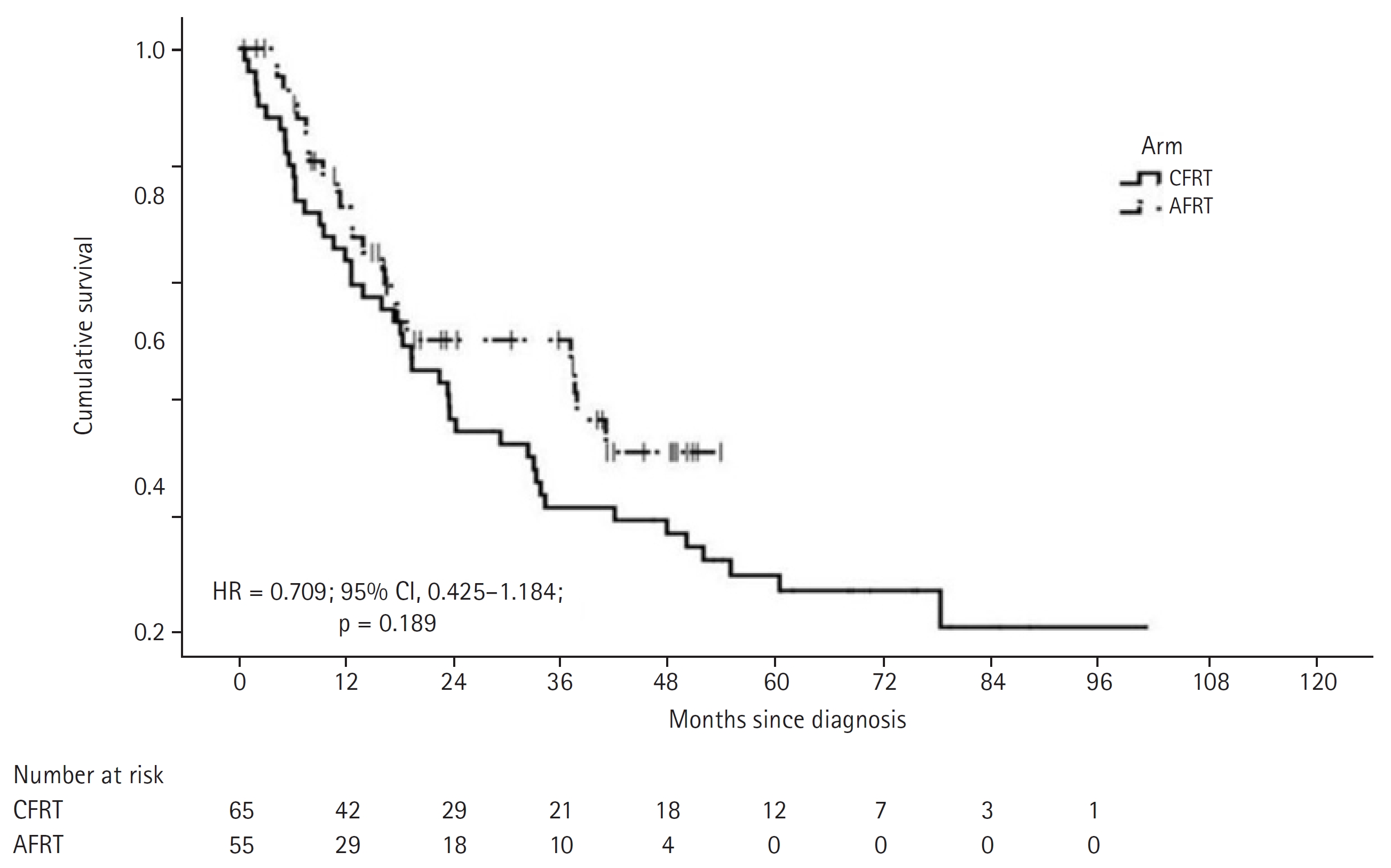
Table 1.Patient and tumour baseline characteristics Values are presented as median (range) or number (%). CFRT, conventional fractionated radiation therapy; AHRT, accelerated hypofractionated radiation therapy; ECOG, Eastern Cooperative Oncology Group; CCI, Charlson Comorbidity Index; PNS, paranasal sinus; CUP, carcinoma of unknown primary; AJCC, American Joint Committee on Cancer. Table 2.Treatment compliance Table 3.Factors affecting overall survival
Table 4.Acute toxicity profile References1. Indian Council of Medical Research; National Centre for Disease Informatics & Research. Clinicopathological profile of cancers in India: a report of the hospital based cancer registries, 2021 [Internet]. Bengaluru, India: Indian Council of Medical Research; 2021 [cited 2023 Aug 20]. Available from: https://ncdirindia.org/All_Reports/HBCR_2021/.
2. Lacas B, Carmel A, Landais C, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol 2021;156:281–93.
3. Muzumder S, Srikantia N, Udayashankar AH, Kainthaje PB, John Sebastian MG. Burden of acute toxicities in head-and-neck radiation therapy: a single-institutional experience. South Asian J Cancer 2019;8:120–3.
4. Muzumder S, Nirmala S, Avinash HU, Kainthaje PB, Sebastian MJ, Raj JM. Early competing deaths in locally advanced head-and-neck cancer. Indian J Palliat Care 2018;24:446–50.
5. Ho KF, Fowler JF, Sykes AJ, Yap BK, Lee LW, Slevin NJ. IMRT dose fractionation for head and neck cancer: variation in current approaches will make standardisation difficult. Acta Oncol 2009;48:431–9.
6. Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–36.
7. Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys 2010;76:1333–8.
8. Daly ME, Le QT, Maxim PG, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 2010;76:1339–46.
9. Overgaard J, Mohanti BK, Begum N, et al. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. Lancet Oncol 2010;11:553–60.
10. Sakso M, Andersen E, Bentzen J, et al. A prospective, multicenter DAHANCA study of hyperfractionated, accelerated radiotherapy for head and neck squamous cell carcinoma. Acta Oncol 2019;58:1495–501.
11. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. Cham, Switzerland: Springer; 2017.
12. Muzumder S, Srikantia N, Udayashankar AH, Kainthaje PB, Sebastian MGJ, Raj JM. Late toxicities in locally advanced head and neck squamous cell carcinoma treated with intensity modulated radiation therapy. Radiat Oncol J 2021;39:184–92.
13. Muzumder S, Srikantia N. Toxicity syndrome and early competing deaths in head-and-neck cancer undergoing radiation therapy: observation and hypothesis. Med Hypotheses 2020;143:110145.
14. Franzese C, Fogliata A, Franceschini D, et al. Impact of hypofractionated schemes in radiotherapy for locally advanced head and neck cancer patients. Laryngoscope 2020;130:E163–70.
15. Deshmukh J, Chatterjee A, Dora TK, et al. Recurrence pattern with respect to two different dose fractionations in patients with locally advanced head and neck cancer treated with chemoradiation using image-guided volumetric arc therapy. Head Neck 2022;44:1690–701.
16. Zamora P, Hammoud A, Dominello MM, Miller SR. Dosimetric comparison of two volume and three volume head and neck treatment plans; is an intermediate risk CTV necessary? Int J Radiat Oncol Biol Phys 2020;108:e365–6.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |