 |
 |
AbstractAlthough Fanconi anemia patients accompany a high risk of multiple cancers, radiation therapy on these patients has been carried out only in limited cases due to the concern for radiation toxicity that stems from their susceptibility to radiation. We report a case of a 28-year-old female patient diagnosed as synchronous esophageal and tongue cancer, and underwent two cycles of radiation therapy, inevitably in the condition of coronavirus disease 2019 infection. She received radiation therapy of 30 Gy to esophageal mass with neoadjuvant aim in her first-round radiation therapy, and later received 27 Gy to tongue cancer surgical bed with adjuvant aim in her second-round radiation therapy. With no further treatment, she has been maintaining no evidence of disease state for 7 months. Managing Fanconi anemia patients with multiple cancers using radiation therapy is feasible, in which cases a dose de-escalation may be important considering the radiation toxicity and possible future re-treatment.
IntroductionFanconi anemia (FA) is a rare congenital syndrome that presents with aplastic anemia and bone marrow hypoplasia in young age and develops myelodysplastic syndrome (MDS) and acute myeloid leukemia during the life course [1]. FA is caused by a defect in a tumor-suppressive pathway, which protects the human genome and maintains genomic stability by repairing DNA interstrand crosslinks [2]. The resulting consequence is skin anomalies including hyperpigmentation and café-au-lait spots (approximately 40%), skeletal anomalies of the head including microcephaly and hydrocephaly (approximately 20%), increased risk of MDS (approximately 6,000-fold greater risk) and leukemia (approximately 700-fold greater risk), increased risk of solid tumor (more than 10% per year by age 45) [3,4]
Another clinical significance of FA patients regarding radiation treatment stems from their inherent sensitivity to DNA damage and the resulting toxicity of varying degrees. Some suggest that the risk of mortality after radiotherapy in FA, pertaining to either acute or chronic radiation toxicity, may amount to as high as 50% [5]. Even those who survive radiotherapy may experience severe local symptoms such as mucositis, dysphagia, stenosis, wound problems, as well as systemic symptoms, the most notable being bone marrow failure. Thus, completion of radiotherapy in an uninterrupted schedule may be limited in many cases, and guidelines recommend radiotherapy to be used only in circumstances of absolute necessity [5,6].
Here, we present a case of a 28-year-old female patient who was diagnosed with synchronous esophageal and tongue cancer and underwent two cycles of radiation therapy due to her disease, inevitably in the condition of coronavirus disease 2019 (COVID-19) infection. She has been maintaining no evidence of disease state for 7 months without severe complication.
Case ReportA 28-year-old female was referred to our hospital due to dysphagia that started 2 weeks earlier. She had underlying FA which was under follow-up at our hospital for 24 years, and a biopsy-proven squamous papilloma of the left tongue that was confirmed 5 years ago.
Computerized tomography (CT) revealed a 3.1-cm-sized enhancing mass in the subcarinal area compressing the esophagus (Fig. 1A, 1B). Esophagogastroduodenoscopy located the mass at 24 cm from the upper incisor (Fig. 1C), and a well-differentiated squamous cell carcinoma (SCC) was confirmed on the biopsy. Positron emission tomography revealed hypermetabolism in the area with no other abnormal lesions (Fig. 1D).
She was advised to receive an upfront surgery owing to the possibility of severe radiation toxicity but was diagnosed with COVID-19. The surgery was withheld because of the grace period required for anesthesia. Thus, a neoadjuvant radiotherapy was planned.
Gross tumor volume (GTV) was defined as the esophageal mass, clinical target volume (CTV) as GTV plus 2 cm margin in superior-inferior and 5 mm margin in radial direction. Planning target volume (PTV) was defined as CTV plus 7 mm margin, and 30 Gy in 15 fractions, 5 fractions per week was prescribed. Treatment planning was performed using the volumetric modulated arc therapy (VMAT) technique (Fig. 2A, 2B).
Before starting the treatment, her performance status measured by the Eastern Cooperative Oncology Group (ECOG) score was 2, and she had Levin-tube in situ for nutrition supply. After successfully finishing the radiotherapy, she reported a slight improvement in the dysphagic symptom and was able to remove the Levin tube, although her ECOG score was still 2.
Two weeks later, she was re-visited with a pneumo-mediastinum, possibly due to a hidden esophageal perforation. She was managed conservatively and received a robot Mckeown operation with three-field lymphadenectomy upon improvement. A 2.4-cm-sized moderately differentiated SCC with clinically negative surgical margin was confirmed. One out of 26 lymph nodes was positive, which was from station #17.
She developed an aspiration tendency during the hospital stay. A left tongue mass near the biopsy-proven squamous papilloma was discovered on work-up. Magnetic resonance imaging (MRI) located a 1.2-cm-sized ovoid well-enhancing mass at the left tongue with no sign of lymph node metastasis (Fig. 3A, 3B). A partial glossectomy was performed, and a 1.6-cm-sized moderately differentiated SCC with a 6.9-mm depth of invasion was confirmed. The surgical margin was clear. Lymph node dissection of 5 left neck level IA/IB nodes identified 1 positive node in level IA, which accompanied extranodal extension. Lymphatic and perineural invasion was present. Subsequently, a post-operative radiotherapy (PORT) with a reduced dose was planned.
CTV-high dose was defined as the surgical bed plus 5 mm margin, CTV-intermediate dose as left neck levels IA/IB/II/III/IV and right neck levels IA/IB/lower II/III, and PTV as CTV plus 3 mm margin. The 54 Gy and 48 Gy was prescribed to PTV-high and PTV-intermediate dose respectively, both in 30 fractions, 5 fractions per week, using the VMAT technique (Fig. 3C, 3D). There was an overlap of low dose region (<5 Gy) with the first-round radiation therapy.
Her ECOG score was 2, evaluated both before and after the treatment. During the radiotherapy, she experienced grade 2 mucositis and moderate odynophagia but managed to maintain oral intake under medication. However, considering her general condition and side effects, the radiation oncologist terminated her treatment after receiving half of the planned dose (27 Gy and 24 Gy). Since then, follow-up evaluation using CT and MRI reveals no evidence of disease (NED) until 7 months period. This study was approved by the Seoul National University Hospital Institutional Review Board (No. H-2307-012-1445). The informed consent was waived.
DiscussionWe report a rare case of FA patient who underwent repeated radiotherapy due to synchronous esophageal and tongue cancer. The patient experienced relatively severe side effects in the second-round radiation therapy and terminated early, but managed to survive until 7 months period. Given the clinical uniqueness of the case and the potential future risks, the patient is scheduled to be followed up every 1–2 months by the relevant department with CT and/or MRI scans being performed every 2–3 months.
Regarding the treatment scheme, the decision to provide radiotherapy was based upon the discussion of esophagus multi-disciplinary team, with reference to the previous reports [1,6-10]. The clinician’s discretion to prescribe low dose in the first-round radiation therapy was a key factor that enabled the second-round of radiotherapy. The early termination of second-round radiation therapy was decided after carefully examining the possible gains and risks of the treatment. At the time of the termination of her second-round radiation therapy, it was evident that she could not receive the remaining planned dose without taking a significant risk of severe side effects, by the time she was already suffering from a grade 2 mucositis. Considering the need for a resting period of at least 2 weeks, an additional gain of delivering the remaining dose was ambiguous. Although the possible gain of early termination of the treatment cannot be fully evaluated at 7 months period post-treatment and the early termination might not have been the best option in terms of local control, the authors believe that there was no negative impact in terms of therapeutic ratio.
One notable characteristic of FA is their high incidence of multiple tumors. One systematic review examined head and neck and esophageal SCC in FA patients and concluded that 9.2% had separate primary tumor in the same region [6]. Another characteristic is their high mortality rate after radiotherapy, which may amount to 50% [5]. Even the survivors may experience severe symptoms such as mucositis, dysphagia, stenosis, and bone marrow failure. Therefore, prudent radiation treatment planning to avoid severe toxicity and allow for possible re-treatments is necessary.
Radiotherapy in FA patients has been tried on limited occasions. The systematic review on FA related head, neck, or esophageal SCC reported that among the 22 patients who received PORT, 11 completed the treatment, and 6 were interrupted [6]. The target dosage was either 60 or 66 Gy, but the median delivered dosage was 51.8 Gy for the reported ones. Radiation was administered once daily schedule in either 1.8 or 2.0 Gy per fraction. In the same study, primary radiotherapy was administered to nine patients, five of whom finished the treatment whereas the remaining four did not. The target dosage was 70 Gy or more, but the average delivered dosage was 52 Gy. As can be seen from this study, it is not uncommon for patients to terminate the treatment early, as was the case in our patient. Because of frequent treatment interruption in FA patients, one study suggested a 4-stage dose and field adjusted approach of 6 weeks, under which radiotherapy was successfully delivered and the toxicity was limited to grade 2–3 mucositis, esophagitis, and nausea [10].
When narrowing the scope to the specific treatment scheme demonstrated in our case, no previous study has reported neoadjuvant radiotherapy alone for esophageal cancer in FA patients, to the best of our knowledge. There was one case where a patient successfully finished neoadjuvant chemoradiotherapy with 30.4 Gy of radiation for esophageal cancer in the middle thoracic area followed by surgery, and remained in NED state until 6 years after surgery [1].
As for tongue cancer, five patients from three previous studies were confirmed, who received adjuvant radiotherapy of 66 Gy, 70.2 Gy, 50 Gy, 25 Gy, and 22 Gy respectively, but died of disease 13, 34, 31, 73, and 5.5 months after surgery or diagnosis. The former four finished the treatment whereas the latter terminated early [7-9]. Previously reported cases of patients who managed to survive for more than 5 years after relatively low doses of radiation (25–30 Gy) are inspiring, as was the case in our patient as well. However, their limitation as case reports should be taken into account and further study is warranted.
Despite the seemingly poor outcomes of radiotherapy in FA patients with cancer and the scarcity of studies on the best treatment options, radiotherapy may play a bigger role in the COVID-19 pandemic era where medical resources are scarce and admissions are avoided [11]. Even so, the risk of patient and/or staff infection as a result of repeat hospital visits and the risk of more serious infection in those receiving radiation therapy should always be considered regarding radiotherapy in COVID-19-positive patients [12]. Nonetheless, our interim success denotes the importance of active treatment for both the multiple cancer patients with FA and COVID-19 patients with cancers. Also, it highlights the importance of dose de-escalation, with the dual purpose of reducing toxicity and reserving doses for the possible future re-treatment.
NotesStatement of Ethics This study was approved by the Seoul National University Hospital Institutional Review Board (No. H-2307-012-1445). Author Contributions Conceptualization, Kim HJ. Investigation and methodology, Kim TH, Kim HJ. Project administration, Kim TH, Kim HJ. Resources, Kim HJ, Kim JH, Kang CH, Keam B. Supervision, Kim HJ. Writing of the original draft, Kim TH. Writing of the review and editing, Kim TH, Kim HJ. Formal analysis, Kim TH, Kim HJ. Data curation, Kim HJ, Kim JH, Kang CH, Keam B. Visualization, Kim TH. All the authors have proofread the final version. Fig. 1.Initial findings of the esophageal cancer on (A) computed tomography, axial plane, (B) computed tomography, coronal plane, (C) esophagogastroduodenoscopy, and (D) positron emission tomography. A 2.4 cm × 1.4 cm × 3.1 cm sized mass is shown in the subcarinal area. 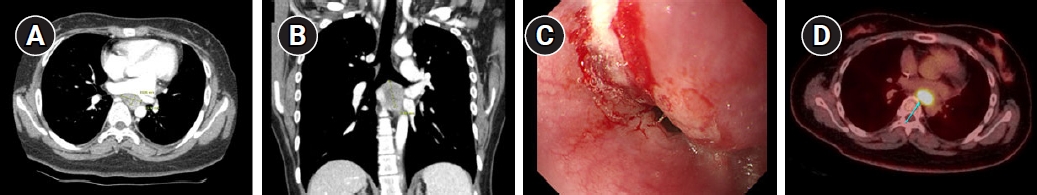
References1. Hosoya Y, Lefor A, Hirashima Y, et al. Successful treatment of esophageal squamous cell carcinoma in a patient with Fanconi anemia. Jpn J Clin Oncol 2010;40:805–10.
2. Kee Y, D’Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev 2010;24:1680–94.
3. Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24:101–22.
4. Alter BP. Fanconi anemia and the development of leukemia. Best Pract Res Clin Haematol 2014;27:214–21.
5. Sroka I, Frohnmayer L, Van Ravenhorst S, Wirkkula L. Fanconi Anemia Clinical Care Guideline, 5th edition [Internet]. Eugene, OR: Fanconi Anemia Research Fund; 2020 [cited 2023 Nov 1]. Available from: https://www.fanconi.org/images/uploads/other/Fanconi_Anemia_Clinical_Care_Guidelines_5thEdition_web.pdf.
6. Lee RH, Kang H, Yom SS, Smogorzewska A, Johnson DE, Grandis JR. Treatment of Fanconi Anemia-associated head and neck cancer: opportunities to improve outcomes. Clin Cancer Res 2021;27:5168–87.
7. Beckham TH, Leeman J, Jillian Tsai C, et al. Treatment modalities and outcomes of Fanconi anemia patients with head and neck squamous cell carcinoma: series of 9 cases and review of the literature. Head Neck 2019;41:1418–26.
8. Kutler DI, Patel KR, Auerbach AD, et al. Natural history and management of Fanconi anemia patients with head and neck cancer: a 10-year follow-up. Laryngoscope 2016;126:870–9.
9. Masserot C, Peffault de Latour R, Rocha V, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer 2008;113:3315–22.
10. Gardner UG, Wood SG, Chen EY, Greenberger JS, Grossberg AJ. Use of a therapeutic trial of graduated neoadjuvant radiation therapy for locally advanced esophageal cancer in a patient with Fanconi anemia. Adv Radiat Oncol 2021;7:100810.
|
|
||||||||||||||||||||||||||||||||||||||||

 |
 |