 |
 |
AbstractPurposeTo investigate the clinical significance of adaptive radiotherapy (ART) in locally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy (IMRT).
Materials and MethodsEligible patients were treated with concurrent chemoradiotherapy using IMRT. Planning computed tomography in ART was performed during radiotherapy, and replanning was performed. Since ART was started in May 2011 (ART group), patients who were treated without ART up to April 2011 (non-ART group) were used as the historical control. The Kaplan-Meier method was used to calculate overall survival (OS), locoregional recurrence-free survival (LRFS), progression-free survival (PFS), and distant metastasis-free survival (DMFS). LRFS for the primary tumor (LRFS_P) and regional lymph node (LRFS_LN) were also studied for more detailed analysis. Statistical significance was evaluated using the log-rank test for survival.
ResultsThe ART group tended to have higher radiation doses. The median follow-up period was 127 months (range, 10 to 211 months) in the non-ART group and 61.5 months (range, 5 to 129 months) in the ART group. Compared to the non-ART group, the ART group showed significantly higher 5-year PFS (53.8% vs. 81.3%, p = 0.015) and LRFS (61.2% vs. 85.3%, p = 0.024), but not OS (80.7% vs. 80.8%, p = 0.941) and DMFS (84.6% vs. 92.7%, p = 0.255). Five-year LRFS_P was higher in the ART group (61.3% vs. 90.6%, p = 0.005), but LRFS_LN did not show a significant difference (91.9% vs. 96.2%, p = 0.541).
IntroductionIn locally advanced nasopharyngeal carcinoma (NPC), concurrent chemoradiotherapy (CCRT) has been the standard treatment because of the tumor's proximity to critical organs such as the brain stem or spinal cord. The widespread use of intensity-modulated radiotherapy (IMRT) in the 2000s enabled uniform dose delivery to the tumor and dose reduction to organs-at-risk (OARs), resulting in improved treatment outcomes and radiation injury-related quality of life (QOL) [1-4]. On the other hand, radiotherapy for head and neck cancers requires approximately 7 weeks of treatment in conventional fractions, and dose uncertainties occur due to weight loss or changes in tumor volume during radiotherapy [5]. Therefore, adaptive radiotherapy (ART) has been widely applied to overcome these difficulties. In ART, planning computed tomography (CT) images are scanned during radiotherapy, and the initial treatment plan is modified to improve the dose distribution [6-8].
Consistent with the advantages of the dosimetric parameters, the effectiveness of ART for NPC has been reported from clinical perspectives [9-11]. A limited number of studies have reported that adaptive therapy improved local control rates but did not affect overall survival. Since the adoption of ART in these studies was determined by patient choice [11] or physician’s decision [9], a certain degree of selection bias might be present. The presence or absence of ART at our institution is determined by the time of treatment. Therefore, we thought that it might be useful to investigate the benefit of ART for NPC using our clinical data. ART has been used since May 2011 in our institutional NPC treatment protocol. In this study, we used patients without ART as the historical control and compared their survival rates to those with ART. This study aimed to investigate the influence of ART on the survival of patients with NPC treated with IMRT.
Materials and Methods1. PatientsThis study was approved by the institutional ethics review committee of Hokkaido University Hospital (No. 21-074). All patients in this study have provided written informed consent for treatment. The included patients had undergone radical radiotherapy for nasopharyngeal cancer between May 2002 and October 2021. Patients treated with CCRT who had completed their scheduled radiation therapy without interruption of more than 2 weeks were eligible. With regard to chemotherapy, patients who received at least one course of CCRT were included, and treatment suspension or discontinuation due to adverse events was allowed. At our institution, ART was initiated in May 2011 (ART group) due to recognition of its utility. In fact, it seems that the importance of ART in IMRT was reported mainly after the first half of 2010 [9,10,12]. To evaluate the influence of ART on survival, we compared patients who received ART with those who did not (non-ART group). The number of eligible cases in the non-ART and ART groups was 26 and 46, respectively (Table 1).
2. ContouringContrast-enhanced CT was used for planning if the patient showed no allergies or other contraindications to the procedure. Enhanced or non-enhanced magnetic resonance imaging (MRI) was performed to assess the extent of the primary tumor or lymph node metastasis. The general contouring procedure was as follows: The gross tumor volume (GTV) was contoured as a primary tumor, and lymph node metastases were identified using available imaging studies, including CT, MRI, and positron emission tomography images. Clinical target volume 1 (CTV1) was basically generated by adding a 5-mm margin to the GTV. CTV1 was modified for areas overlapping air or outside the body. CTV2 was generated as a high-risk region where the tumor was potentially present, excluding CTV1. CTV3 was also generated as an intermediate- or low-risk region, which included the whole-neck regions, excluding CTV1 and CTV2. Planning target volume 1 (PTV1) was generated by adding a 3- to 5-mm margin to the CTV1. PTV2 was generated by adding a 3- to 5-mm margin to CTV2, excluding PTV1. Similarly, PTV3 was generated as CTV3 with a 3- to 5-mm margin, excluding PTV1 and PTV2.
3. RadiotherapyDoses of 66–71 Gy were administered in all cases as radical treatment (Table 2). The general dose prescription was 70 Gy in 35 fractions for 45 patients (97.8%) in the ART group and 18 patients (69.2%) in the non-ART group (Table 2). In patients receiving 70 Gy in 35 fractions using the simultaneous integrated boost (SIB) IMRT method, 70 Gy, 63 Gy, or 54 Gy were prescribed to 95% of the PTV1, PTV2, and PTV3, respectively. In patients receiving 66 Gy, these doses were prescribed to 95% of the PTV1, and 50.4–56 Gy was prescribed to 95% of PTV2 and PTV3. Using the sequential boost (SEQ) IMRT method, 46 Gy was prescribed to all regions of PTV1, PTV2, and PTV3, and 24 Gy was subsequently prescribed to the reduced region, including the original GTV. Since previous randomized controlled trials have reported no difference in treatment outcomes between SIB and SEQ-IMRT, they were analyzed together in this study [13]. In cases where the PTV was in contact with a critical organ, such as the brainstem or spinal cord, priority was given to achieving dose constraints for OARs. The dosimetric data of initial IMRT plans are shown in Supplementary Table S1.
IMRT was performed by the step-and-shoot technique until January 2019 and by volumetric modulated arc therapy after that. XiO (Elekta AB, Stockholm, Sweden), Pinnacle3 (Philips, Amsterdam, The Netherlands) or Raystation (RaySearch Laboratories AB, Stockholm, Sweden) was used as the treatment planning system. With regard to the linear accelerator (LINAC), MHCL-15SPLINAC (Mitsubishi Electronics Co. Ltd., Tokyo, Japan) was used until January 2012, Varian CLINIC iX (Varian Medical Systems, Palo Alto, CA, USA) was used from February 2012 to March 2019, and TrueBeam (Varian Medical Systems) was used subsequently. Almost all patients were treated with 6-MV X-rays, except one patient who received treatment with 4-MV X-rays.
4. Adaptive radiotherapyThe goal of ART is to correct dose uncertainties via one or more replanning sessions during radiotherapy, thereby optimizing the delivered dose distribution to the patient's anatomy [14]. At our institution, planning CT is typically performed once during the course of radiotherapy, and replanning is performed as needed. We defined the CT images acquired in the middle of radiotherapy as the 2nd planning CT. The 2nd planning CT images (CT_2nd) were obtained at a median of 19.5 days (interquartile range, 16 to 21 days) after the treatment initiation date. The target and OAR contours were generated in the same manner as in the initial treatment plan, and the IMRT plan was then regenerated to adhere to the dose constraint for the total dose. The adaptive plan was started at 46 Gy. In recently treated cases, dose distributions of the initial IMRT plan were evaluated on CT_2nd to determine the necessity for replanning. The beam data of the initial plan were transferred and calculated on CT_2nd, and dosimetric parameters were reviewed (Supplementary Table S2). The general criteria for replanning were as follows: the dose constraints of targets and serial organs (i.e., spinal cord or brain stem) were not met, and the key dose parameters of parallel organs were increased by more than 5% in comparison with the initial plan. A summary of the ART flow is shown in Fig. 1.
5. ChemotherapyAll patients received at least one course of concurrent chemotherapy (Table 2). Chemotherapy regimens were generally tri-weekly cisplatin (CDDP) 80 mg/m2 for three courses or weekly CDDP 40 mg/m2 for six courses during CCRT. Induction or adjuvant chemotherapy was administered to patients with advanced disease, based on the treatment strategy determined at a multidisciplinary cancer conference. The general regimen of induction chemotherapy consisted of three cycles of TPF (docetaxel 75 mg/m2, cisplatin 75 mg/m2, and 5 continuous days of fluorouracil 750 mg/m2), and the adjuvant regimen involved three cycles of FP (cisplatin 70 mg/m2 and 5 continuous days of fluorouracil 700 mg/m2). Because a CDDP dose of 200 mg/m2 during CCRT is a critical criterion to predict survival benefits in patients receiving CCRT for NPC [15,16], 200 mg/m2 was used as the cutoff value in patients receiving weekly or tri-weekly CDDP.
6. Follow-upAll patients were evaluated weekly during radiotherapy and then until recovery from acute radiation reactions. Follow-up assessments were scheduled every 1–3 months for the first year, depending on the patient's condition, 3–4 months for the second and third years, and 6–12 months thereafter. Laryngoscopy was performed at every follow-up visit, and imaging assessments by CT or MRI were performed on most follow-up days. To evaluate the patients’ QOL, we routinely used several questions (swallowing, sensory problems, and dry mouth) from the European Organization for Research and Treatment of Cancer (EORTC) Head and Neck Quality of Life Questionnaire 35 (QLQ-H&N35) [17]. For problems regarding sense, only taste was assessed. A survey on the last follow-up day was reviewed and analyzed.
7. Statistical analysisThe primary endpoint was overall survival (OS), which was calculated from the initial day of radiotherapy to the last follow-up day or death. The secondary endpoints were locoregional recurrence-free survival (LRFS), progression-free survival (PFS), and distant metastasis-free survival (DMFS). These survival rates were calculated as the day of locoregional (primary tumor and lymph node region) recurrence, any recurrence, or distant metastasis, respectively. If the patient showed no evidence of recurrence, the calculation was performed up to the last follow-up day. For assessment of LRFS, we also investigated locoregional recurrence-free survival of primary tumors (LRFS_P) and regional lymph nodes (LRFS_LN). Kaplan-Meier methods were used to calculate survival rates, and statistical significance was evaluated using the log-rank test. Fisher' exact test or chi-square test was used to analyze categorical variables, and the Mann-Whitney U test was used for continuous variables. Statistical significance was set at p < 0.05, and these analyses were performed using JMP Pro version 16 (SAS, Cary, NC, USA).
Results1. Patients and characteristicsPatients were categorized into three subgroups based on histology according to the World Health Organization classification: type 1, keratinizing squamous cell carcinoma (13.9%); type 2, differentiated non-keratinizing carcinoma (25.0%); and type 3, undifferentiated non-keratinizing carcinoma (61.1%). The ART and non-ART groups showed no significant differences in patient background factors, except sex and body mass index (BMI) (Table 1). In the non-ART and ART groups, radiation doses were at least 66 Gy in all cases, although statistical analysis based on 70 Gy showed a higher proportion of those who received ≥70 Gy in the ART group (Table 2).
2. Survival analysisThe median follow-up period was 127 months (range, 10 to 211 months) in the non-ART group and 61.5 months (range, 5 to 129 months) in the ART group. The 5-year survival rates in the non-ART and ART groups were as follows: OS was 80.8% (95% confidence interval [CI], 61.3–91.8) and 80.7% (95% CI, 64.1–90.7; p = 0.941), PFS was 53.8% (95% CI, 35.0–71.6) and 81.3.% (95% CI, 66.7–90.4; p = 0.015), LRFS was 61.2% (95% CI, 41.7–77.8) and 85.3% (95% CI, 70.8–93.3; p = 0.024), and DMFS was 84.6% (95% CI, 65.5–93.6) and 92.7% (95% CI, 79.6–97.6; p = 0.255), respectively (Fig. 2). Analysis limited to patients receiving 70 Gy or higher doses also showed better 5-year LRFS in the ART group (85.3%; 95% CI, 70.8–93.3) than in the non-ART group (55.0%; 95%CI, 32.3–75.8%; p = 0.011).
In a detailed analysis of LRFS, the 5-year LRFS-P was 61.3% (95% CI, 41.7–77.8) and 90.6% (95% CI, 77.5–96.4; p = 0.005), and LRFS_LN was 96.2% (95% CI, 77.2–99.5) and 91.9% (95% CI, 77.7–97.4; p = 0.541) (Fig. 3) in the non-ART and ART groups, respectively.
3. Failure patternsHigher recurrence rates were observed in the non-ART group than in the ART group (50.0% vs. 17.3%) (Table 3). Locoregional recurrence was found in 6 (13.0%) of 46 patients who received ≥70 Gy in the ART group and 8 (44.4%) of 18 patients who received 70 Gy and 3 (37.5%) of 8 patients who received 66 Gy in the non-ART group.
4. QOL scoresA part of the EORTC QLQ-H&N35 scores (swallowing, sensory problems, and dry mouth) was available from 49 patients (non-ART group of 10 patients; ART group of 39 patients). The mean ± standard deviation dry mouth score was higher in the ART than in the non-ART group (31.6 ± 29.6 vs. 13.3 ± 23.3), but the difference was not significant (Table 4). No significant differences were found between the two groups in swallowing and taste. In dosimetric evaluation during radiotherapy using CT_2nd, no significant dose increase in OAR was observed (Supplementary Table S2).
Discussion and ConclusionThis study investigated the impact of ART on treatment outcomes in 72 patients with locally advanced NPC. Overall, ART showed a notable benefit for LRFS but not for OS or DMFS. More detailed analyses showed that ART did not change LRFS_LN but significantly improved LRFS_P. The results of the present study provide essential insights into the potential effectiveness of ART for primary tumor control. On the other hand, since the presence or absence of ART was determined by the time of treatment, differences in patient background between the ART and non-ART groups should be noted.
In a comparison of the ART and non-ART groups, LRFS was higher in the ART group, but no significant differences were observed in OS or DMFS. These trends are very similar to those reported in previous studies. Luo et al. studied the effectiveness of adaptive replanning methods in 132 patients with locally advanced NPC using propensity-score matching and reported that replanning was a prognostic factor for LRFS, but not for DMFS, PFS, and OS [9]. For 290 NPC patients treated by IMRT with or without replanning, Zhou et al. [11] reported that the replanning group showed a superior 8-year LRFS (87.4% vs. 75.6%, p = 0.025). However, the 8-year OS (60.9% vs. 59.4%, p = 0.701) and DMFS (82.3% vs. 76.8%, p = 0.378) were not significantly different in the groups with and without replanning. As for the reason behind the lack of a difference in OS between both groups, they suggested that a certain percentage of NPCs have potentially distant metastases, and it is difficult to reduce these risks by replanning. In our review of relapsed cases, many patients had longer tumor control with chemotherapy and local radiotherapy, a possible reason behind the lack of a difference in OS. These results indicate that the benefit of ART was mainly in improving local and regional control rates.
In comparison with the non-ART group, the ART group showed a significantly higher LRFS_P, although the LRFS_LN was not significantly different. Studies investigating LRFS_P and LRFS_LN separately are limited, but a similar trend was found in a previous study. In the study by Luo et al., which has been mentioned above, the lymph node recurrence rate was 3.0% (two patients) in the replanning group and 1.5% (one patient) in the non-replanning group. In contrast, the primary tumor recurrence rate reduced from 6.1% (four patients) without replanning to 0% with replanning [9]. Although no statistical analysis was performed separately between the regional lymph node and primary tumor, their study suggested that the benefit of replanning may be greater in reducing primary tumor recurrence. Thus, when considering CT replanning during the course of treatment, changes in dosimetric parameters at the primary site may be more important.
The reasons for the higher LRFS_P with ART observed in the present study require further investigation. Although several studies have reported the benefit of ART in locally advanced NPC from the viewpoint of dosimetric parameters, they did not reveal any trend differences between the findings for the primary tumor and lymph nodes. Cheng et al. [5] studied 19 patients who underwent IMRT for locally advanced NPC and were prospectively imaged using CT at 30 Gy and 50 Gy. Using CT images obtained during radiotherapy, they showed that replanning at 30 Gy was essential to maintain a satisfactory dose to the target volumes. However, the benefit of replanning was observed in both primary tumors and lymph node metastases. By comparing the findings between the non-ART plan and ART plan in NPC, Deng et al. [18] also suggested that ART resulted in a significant improvement in the conformity index for both the primary disease and the involved lymph node. In the present study, the baseline GTV volume was greater for primary tumors than that for lymph node metastases in the ART and non-ART groups (p < 0.05). Therefore, the difference in the baseline tumor volume may have influenced the results.
Another hypothesis to explain the higher LRFS_P in the ART group may be attributed to a slight difference in the prescribed radiation dose or CDDP dose between the non-ART and ART groups. In this study, a higher proportion of patients in the ART group were treated with 70 Gy. In contrast, Wang et al. [19] showed that the locoregional control in at least T1-T3 NPC was not significantly different between IMRT with 70 Gy and 63.6 Gy (median dose), although around one-third of the patients in that study received radiotherapy alone. In this study, locoregional recurrences were found in 8 of 18 (44.4%) patients in the 70 Gy dose subgroup and 3 of 8 (37.5%) patients in the 66 Gy dose subgroup in the non-ART group. There was no clear association between the prescribed dose and the locoregional recurrence rate, although the small sample size makes it difficult to draw definitive conclusions. As shown in Table 1, the CDDP dose tended to be higher in the ART group, although its significance was borderline. This difference may be attributed to the possibility of subtle changes in the CDDP administration policy by treatment year or improvement in supportive care in recent years, including nutritional management.
There were no significant differences in EORTC QLQ-H&N35 scores between the non-ART and ART groups. Although only seven cases were evaluated for dosimetric parameters of the initial IMRT plan using CT_2nd, no significant dose increase in OAR was found in the dosimetric analysis during radiotherapy (Supplementary Table S2). The lack of difference in QOL scores between the non-ART and ART groups is consistent with the dosimetric analysis. However, it is difficult to draw definitive conclusions due to the limited number of cases. Moreover, the questionnaire used in this study was not comprehensive, and the number of surveyed patients was limited. These limitations may have led to the lack of significant differences in QOL scores between the non-ART and ART groups. Nonetheless, a study with a large cohort of NPC showed that replanning improved QOL scores for dry mouth and sticky saliva [10,11]. Studies on the influence of ART on QOL scores are limited, and further studies are needed.
The limitations of this study are as follows. The influence of the treatment year cannot be ruled out because the time period of this study that used a historical control is more than 15 years. As noted above, the prescribed dose and CDDP dose during CCRT tended to be higher in the ART group, which may have contributed to the better locoregional control rate in the ART group to some extent. The differences in the LINAC, accuracy of patient setup, and experiences in IMRT planning may have affected the results of radiotherapy. Another limitation was the significant sex-related difference between the non-ART and ART groups (p < 0.001). The ART group contained significantly more male patients than the non-ART group, but the reason for this difference was unknown. This may be a coincidence because radiotherapy with or without ART depends only on the year of treatment. Since several studies reported that male sex was an adverse prognostic factor for PFS or LRFS [1,20,21], the high ratio of men in the ART group had a negative impact on the treatment outcomes. However, the ART group showed better PFS and LRFS compared to the non-ART group, which suggests that the sex ratio imbalance between the two groups may not be related to the conclusions of the present study. Moreover, the fact that men have a higher BMI in the general population may also explain the significantly higher baseline BMI in the ART group compared to that in the non-ART group [22].
In conclusion, the present study suggests that ART may improve LRFS in NPC, similar to the findings reported by previous studies. A new finding of this study is that LRFS_P was improved by ART more than LRFS_LN. However, it should be noted that there were some differences in patient backgrounds between the ART and non-ART groups. The effectiveness of ART in NPC needs to be further explored by analysis of larger populations with more homogeneous patient backgrounds.
NotesStatement of Ethics This study was approved by the institutional ethics review committee (IRB number: 21-074). All patients have provided written informed consent for treatment. Consent to participate in this study was obtained in the form of opt-out on the website. Conflict of Interest K.K. is an employee of the research institute of Hitachi, Ltd., currently working for Hokkaido University under a secondment agreement. K.K. declares that this research has no relationship to Hitachi, Ltd. All other authors declare that they have no conflicts of interest to declare. Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2023.00374.
Supplementary Table S1.Dosimetric parameters of the target volumes of the non-ART and ART groups Supplementary Table S2.Dosimetric parameters of the target volumes for the 1st plan and 1st plan on CT_2nd (n = 7) Fig. 1.The diagram of adaptive radiotherapy. All patients in the non-ART group completed scheduled IMRT using the initial treatment plan. In dosimetric evaluation during radiotherapy, the beam data of the initial plan was transferred and calculated on the 2nd planning CT, after which dosimetric parameters were reviewed. Here, 2nd planning CT means CT images acquired in the middle of radiotherapy. In the ART group, 41 cases were replanned, and five patients continued the initial IMRT plan after dose evaluation of the initial plan. ART, adaptive radiotherapy; IMRT, intensity-modulated radiotherapy; CT, computed tomography. 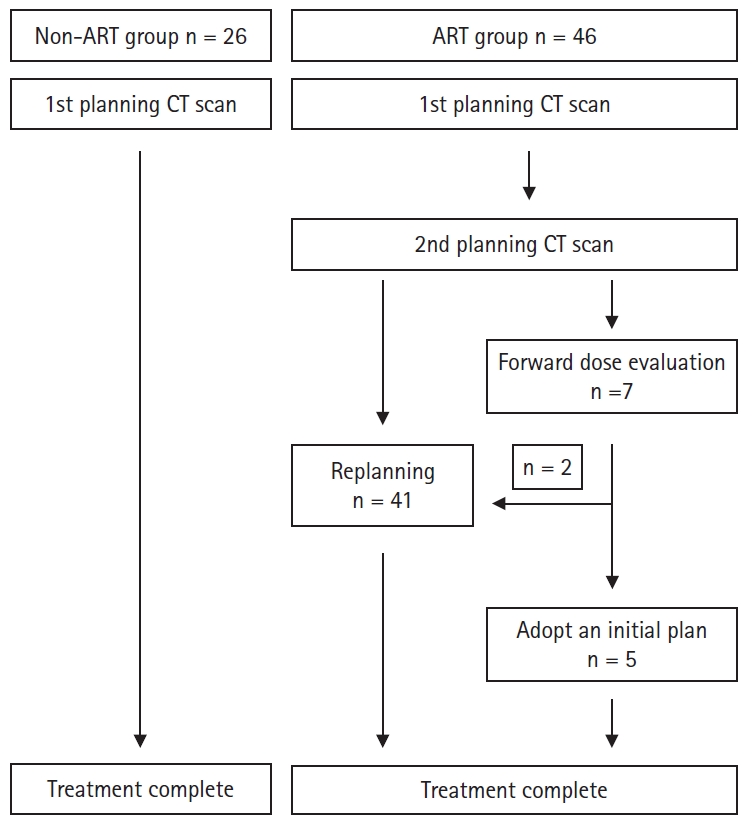
Fig. 2.(A) Overall survival, (B) progression-free survival, (C) locoregional recurrence-free survival, and (D) distant metastasis-free survival between the non-ART (blue) and ART (red) groups. ART, adaptive radiotherapy. 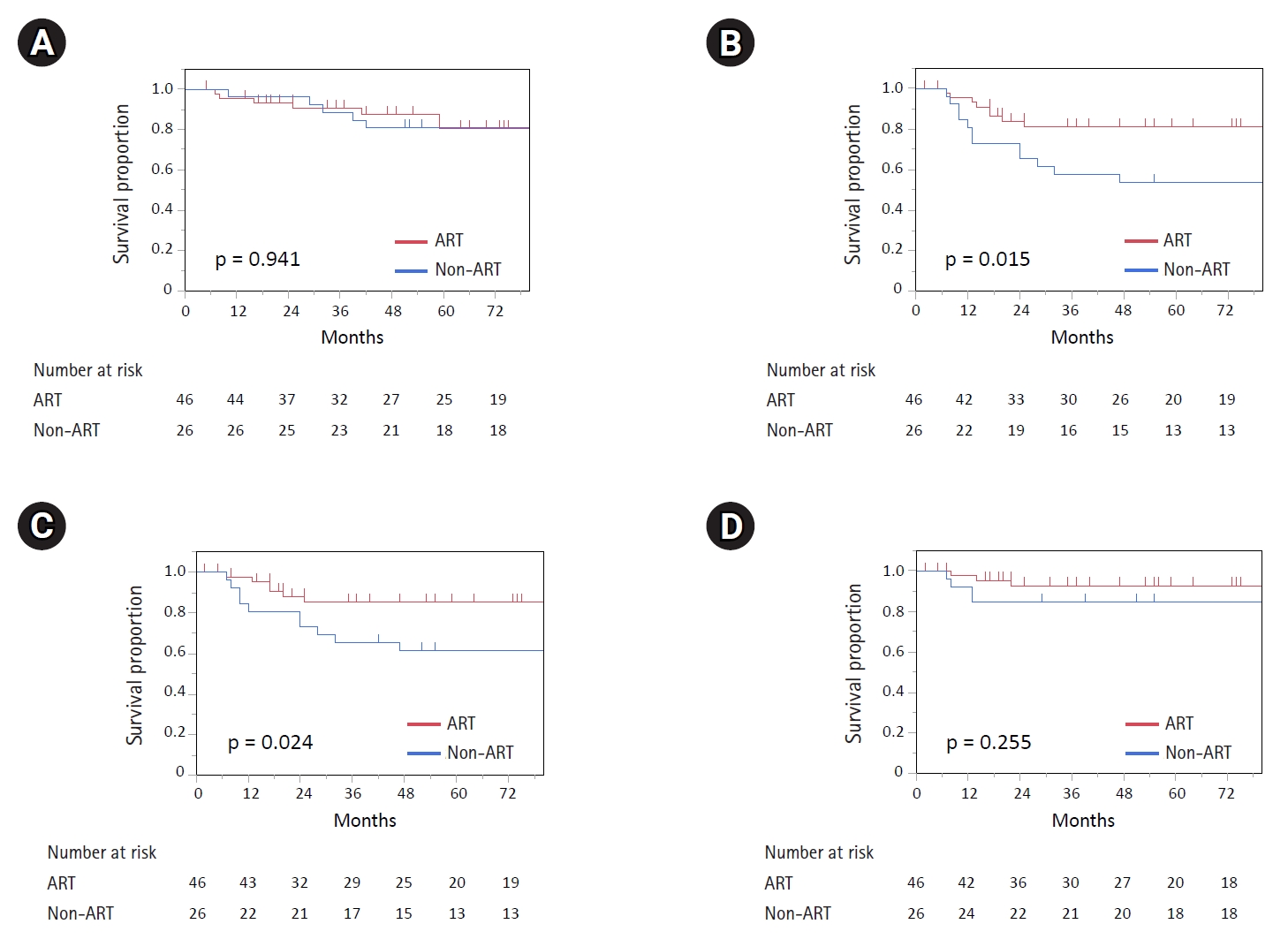
Fig. 3.(A) Locoregional recurrence-free survival of primary tumor and (B) locoregional recurrence-free survival of the regional lymph node between the non-ART (blue) and ART (red) groups. ART, adaptive radiotherapy. 
Table 1.Patient background data
Values are presented as number (%) or median (range) or mean ± standard deviation. ART, adaptive radiotherapy; WHO, World Health Organization; UICC, Union for International Cancer Control; CDDP, cisplatin; BMI, body mass index; GTV, gross tumor volume; IQR, interquartile range. Table 2.Chemotherapy and radiotherapy regimens
Table 3.Patterns of failure Table 4.EORTC QLQ-H&N35 scores in the non-ART and ART groups
References1. Sun XS, Liu SL, Luo MJ, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys 2019;105:581–90.
2. Gupta T, Sinha S, Ghosh-Laskar S, et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: long-term and mature outcomes of a prospective randomized trial. Radiat Oncol 2020;15:218.
3. Fang FM, Chien CY, Tsai WL, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy: a longitudinal study. Int J Radiat Oncol Biol Phys 2008;72:356–64.
4. Tribius S, Bergelt C. Intensity-modulated radiotherapy versus conventional and 3D conformal radiotherapy in patients with head and neck cancer: is there a worthwhile quality of life gain? Cancer Treat Rev 2011;37:511–9.
5. Cheng HC, Wu VW, Ngan RK, et al. A prospective study on volumetric and dosimetric changes during intensity-modulated radiotherapy for nasopharyngeal carcinoma patients. Radiother Oncol 2012;104:317–23.
6. Lu J, Ma Y, Chen J, et al. Assessment of anatomical and dosimetric changes by a deformable registration method during the course of intensity-modulated radiotherapy for nasopharyngeal carcinoma. J Radiat Res 2014;55:97–104.
7. Kanehira T, van Kranen S, Jansen T, et al. Comparisons of normal tissue complication probability models derived from planned and delivered dose for head and neck cancer patients. Radiother Oncol 2021;164:209–15.
8. Ghosh A, Gupta S, Johny D, Vidyadhar Bhosale V, Pal Singh Negi M. A study to assess the dosimetric impact of the anatomical changes occurring in the parotid glands and tumour volume during intensity modulated radiotherapy using simultaneous integrated boost (IMRT-SIB) in head and neck squamous cell cancers. Cancer Med 2021;10:5175–90.
9. Luo Y, Qin Y, Lang J. Effect of adaptive replanning in patients with locally advanced nasopharyngeal carcinoma treated by intensity-modulated radiotherapy: a propensity score matched analysis. Clin Transl Oncol 2017;19:470–6.
10. Yang H, Hu W, Wang W, et al. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2013;85:e47–54.
11. Zhou X, Wang W, Zhou C, et al. Long-term outcomes of replanning during intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: an updated and expanded retrospective analysis. Radiother Oncol 2022;170:136–42.
12. Chen AM, Daly ME, Cui J, et al. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck 2014;36:1541–6.
13. Lertbutsayanukul C, Prayongrat A, Kannarunimit D, et al. A randomized phase III study between sequential versus simultaneous integrated boost intensity-modulated radiation therapy in nasopharyngeal carcinoma. Strahlenther Onkol 2018;194:375–85.
14. Castelli J, Simon A, Lafond C, et al. Adaptive radiotherapy for head and neck cancer. Acta Oncol 2018;57:1284–92.
15. Peng H, Chen L, Zhang Y, et al. Prognostic value of the cumulative cisplatin dose during concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a prospective phase III clinical trial. Oncologist 2016;21:1369–76.
16. Guo SS, Tang LQ, Zhang L, et al. The impact of the cumulative dose of cisplatin during concurrent chemoradiotherapy on the clinical outcomes of patients with advanced-stage nasopharyngeal carcinoma in an era of intensity-modulated radiotherapy. BMC Cancer 2015;15:977.
17. Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 1999;17:1008–19.
18. Deng S, Liu X, Lu H, et al. Three-phase adaptive radiation therapy for patients with nasopharyngeal carcinoma undergoing intensity-modulated radiation therapy: dosimetric analysis. Technol Cancer Res Treat 2017;16:910–6.
19. Wang X, Wang Y, Jiang S, et al. Safety and effectiveness of de-escalated radiation dose in T1-3 nasopharyngeal carcinoma: a propensity matched analysis. J Cancer 2019;10:5057–64.
20. Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018;77:16–21.
|
|

 |
 |