Radiotherapy combined with immunotherapy could improve the immune infiltration of melanoma in mice and enhance the abscopal effect
Article information
Abstract
Purpose
To analyze the gene mutation, immune infiltration and tumor growth of primary tumor and distant tumor under different treatment modes.
Materials and Methods
Twenty B16 murine melanoma cells were injected subcutaneously into the of both sides of the thigh, simulating a primary tumor and a secondary tumor impacted by the abscopal effect, respectively. They were divided into blank control group, immunotherapy group, radiotherapy group, and radiotherapy combined immunotherapy group. During this period, tumor volume was measured, and RNA sequencing was performed on tumor samples after the test. R software was used to analyze differentially expressed genes, functional enrichment, and immune infiltration.
Results
We found that any treatment mode could cause changes in differentially expressed genes, especially the combination treatment. The different therapeutic effects might be caused by gene expression. In addition, the proportions of infiltrating immune cells in the irradiated and abscopal tumors were different. In the combination treatment group, T-cell infiltration in the irradiated site was the most obvious. In the immunotherapy group, CD8+ T-cell infiltration in the abscopal tumor site was obvious, but immunotherapy alone might have a poor prognosis. Whether the irradiated or abscopal tumor was evaluated, radiotherapy combined with anti-programmed cell death protein 1 (anti-PD-1) therapy produced the most obvious tumor control and might have a positive impact on prognosis.
Conclusion
Combination therapy not only improves the immune microenvironment but may also have a positive impact on prognosis.
Introduction
Melanoma is a skin cancer caused by malignant melanocytes and is highly malignant [1]. In recent years, its incidence rate has increased sharply, and this increase in rate is faster than that of other types of cancer, which has become a thorny public health problem [2,3]. Several subtypes of melanoma have been defined, including uveal, mucosal, meningeal, anorectal and the most common type, cutaneous. The differences in prognosis among these subtypes mainly depend on the stage at diagnosis [3,4]. Early diagnosis is crucial for improving prognosis because most of the complications of melanoma come from metastasis and its impact on the affected organs [5].
To treat local or metastatic tumors, the primary melanoma and metastatic tumors should be surgically removed as completely as possible for therapeutic purposes [6]. In addition, melanoma is an immunogenic tumor [7], and the efficacy of immune checkpoint inhibitors has been shown to be superior to that of conventional drugs. At present, nivolumab monotherapy, pembrolizumab monotherapy, and nivolumab combined with ipilimumab are the first choices for systemic immunotherapy for patients with advanced melanoma [8-10]. However, less than 50% of patients show a persistent antitumor response and benefit from immunotherapy alone, and a considerable proportion of patients show drug resistance [11,12].
Radiotherapy is usually considered a local treatment for cancer. However, in recent years, an increasing number of studies have shown that radiotherapy can induce tumor regression outside the irradiation field in solid cancers. This systemic response is called the abscopal effect [13]. The occurrence of the abscopal effect is related to the specific immune activation caused by radiation-induced cell death [14]. Before the advent of immunotherapy, a review of the abscopal effect of radiotherapy showed that only 46 cases of the abscopal effect were reported among the millions of patients receiving treatment [15]. Immune checkpoint inhibitors can enhance the function of T cells, so an increasing number of studies have evaluated the effect of combining immunotherapy and radiotherapy on primary and abscopal tumors. During immunotherapy, the observation of an abscopal response in patients receiving palliative radiotherapy due to tumor progression is helpful for understanding the immunogenicity of radiotherapy and the potential mechanism of its interaction with cancer immunotherapy [16,17]. The results have shown that the addition of immunotherapy significantly enhances the abscopal effect-induced impact of radiotherapy and that radiotherapy may increase the immunogenicity of tumors and reprogram them [14,18].
The purpose of our study is to analyze the gene expression in primary tumor and abscopal tumor from a genetic perspective and the effect of different treatment methods on immune infiltration and prognosis by establishing a mouse abscopal tumor model with the help of R language (https://www.r-project.org/).
Materials and Methods
1. Mice
Twenty C57BL/6 mice (6 to 10 weeks of age) with the same mouse source were obtained from Cavens Laboratory Animal Co., Ltd. Mice were treated according to a protocol approved by the Animal Care and Use Committee of Soochow University (No. SUDA20221024A02). While housed on a 12-hour light/dark cycle, mice were given free access to food and water.
2. Animal model construction
After 7 days of feeding, 5 × 105 B16 cells in 1,250 μL phosphate-buffered saline (PBS) were subcutaneously injected into the right thigh of mice to simulate a primary tumor (irradiated tumor), and 3 × 105 B16 cells in 750 μL PBS were injected under the skin of the left thigh to simulate a secondary tumor (abscopal tumor), this timepoint was considered day 0. The B16-F10 mouse melanoma cell line were purchased from BeNa Culture Collection (Suzhou, China).
When the tumor volume reached 150 mm3, the mice were randomly divided into four groups: control group, immunotherapy group, radiotherapy group, and radiotherapy combined with immunotherapy group. The immunotherapy scheme was to intraperitoneally inject anti-mouse programmed cell death protein 1 (PD-1) (10 mg/kg) five times [19], specifically on the 10th, 12th, 14th, 16th, and 18th days of the experiment. Purified anti-mouse PD-1 was purchased from Shanghai Junshi Biological Science Co., Ltd. For the radiotherapy group, on the 14th day, the right thigh of each mouse was irradiated with a single dose of 20 Gy of 6 MeV electron rays from the Elekta Infinity linear accelerator. The irradiation field was 20 cm × 5 cm and the SSD was 100 cm. Irradiate five mice side by side each time. The control group did not receive any treatment.
3. Tumor growth measurement
Tumor volume was measured every 2 days by an electronic caliper, and the following formula was used to calculate the volume:
Volume = π/6 × ab2,
where a and b in the formula are the long and short axes of the two orthogonal diameters, respectively.
4. Survival rate assessment
Mice were monitored daily to assess their survival over a 20-day period. Survival time was recorded as the time until the date of death. Mice were euthanized by carbon dioxide gas asphyxiation when the experiment was over or when they exhibited signs of poor body condition following the Institutional Animal Care policy and guidelines approved by the American Veterinary Medical Association.
5. RNA sequencing
At the end of the experiment, one mouse in each group was selected for RNA sequencing of its primary tumor and secondary tumor (all mice come from the same mouse source, and each group of mice receives the same treatment mode). All sequencing was conducted on an Illumina HiSeq Platform (Illumina Inc., San Diego, CA, USA) with 200-bp paired-end reads following the manufacturer’s protocol. Initial quality control of the RNA sequencing data was performed with the FastQC tool. For each sample, STAR aligner was used to align the short reads to the GRCm38/mm10 reference genome. For the expression of mRNA transcripts, normalized fragments per kilobase per million mapped reads (FPKM) were obtained using the robust FPKM estimate function of the DESeq2 tool.
6. Bioinformatic analysis
For human analyses, the mouse gene IDs were converted to human gene IDs via R package “biomaRt.” For mRNA transcripts, DESeq software was used to screen known transcripts differentially expressed between different sample groups; differentially expressed genes met the threshold of |log2FC| ≥ 1 and p ≤ 0.05. A volcano plot and heatmap were used to visualize the different genes in different samples.
For analyzing the immune cell types in all of the sample genes, CIBERSORT (http://cibersort.stanford.edu/) was used to calculate the composition of immune cells from gene expression profiles. All gene expression matrix from eight samples were imported in CIBERSORT website, CIBERSORT’s LM22 signature matrix was used as the reference to estimate the proportions of nine kinds of immune cells in the RNA-seq samples. R package "ESTIMATE" was used to predict tumor purity.
The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed using the clusterProfiler package in R. The GO terms and KEGG pathways were considered statistically significant if their p-values and false discovery rates (FDR) were less than 0.05.
7. Statistical analysis
R software (version 4.1.0) was used to process data, and R language was used for visualization. GraphPad 8.0 software was also used for visualization, and data are presented as the mean ± standard error or mean ± standard deviation as indicated. The difference between two groups of samples passed the Wilcoxon test. p < 0.05 was considered statistically significant.
Results
1. Difference in gene expression profiles between irradiated tumors and abscopal tumors
To fully reflect the advantages of radiotherapy combined with anti-PD-1 immunotherapy versus the other three treatment modes, one mouse was selected from each of the four groups on the 20th day of the experiment, and the primary tumor and secondary tumor were isolated. A total of eight samples were sent for RNA sequencing. Volcano plot results showed that compared with the control group, the combination treatment group contained 641 differentially expressed genes, including 92 genes that were upregulated and 549 genes that were downregulated (Fig. 1A). There were 170 differentially expressed genes between the immunotherapy only group and the combination treatment group, including 50 genes that were upregulated and 120 genes that were downregulated (Fig. 1B). Compared with the radiotherapy alone group, the combination treatment group contained 33 genes that were upregulated and 86 genes that were downregulated, with a total of 119 differentially expressed genes (Fig. 1C). In addition, we found that both irradiated tumors and abscopal tumors could have differential gene changes, but these were mainly found in the irradiated tumors (Fig. 1D–1F). In short, whether irradiated or abscopal tumors are assessed, there will be changes in differential genes.
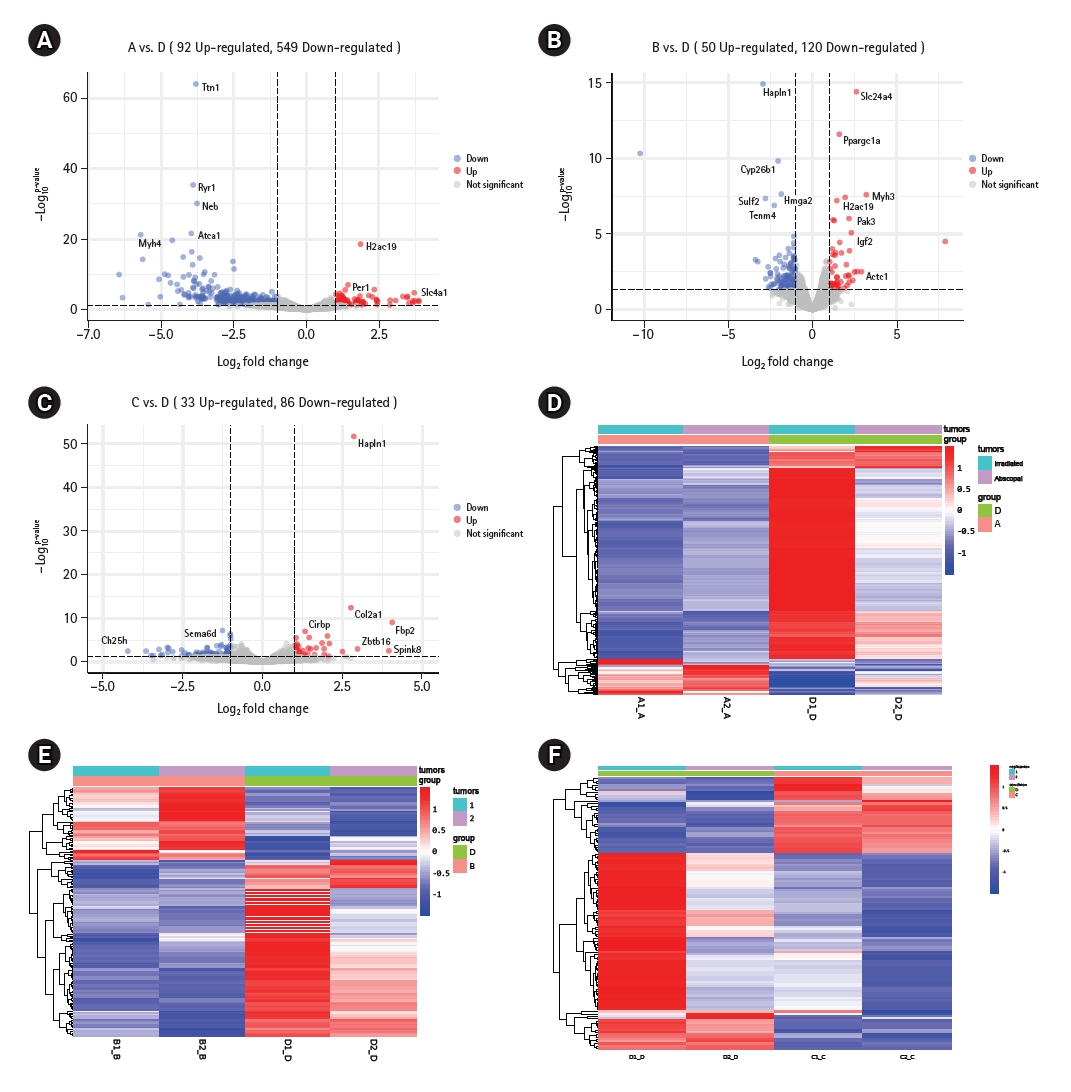
Genes change differently under different treatment modes. (A–C) The volcanic maps show the differences in gene expression between the combination therapy group and the control group, immunotherapy group, and radiation therapy group, respectively. (D–F) The thermograms show the differential genes between irradiated tumor and abscopal tumor "A" represents blank control group, "B" represents simple immunotherapy group, "C" represents radiotherapy group, "D" represents radiotherapy combined immunotherapy group, "1" represents primary tumor, and "2" represents secondary tumor (the same below). p < 0.05 was statistically significant.
2. Radiotherapy combined with anti-PD-1 therapy changed the enriched pathways of differential genes between irradiated and abscopal tumors
Next, we further compared the pathways related to differential gene enrichment between radiotherapy combined with anti-PD-1 therapy and the other three groups. We first conducted GO enrichment analysis, and the results showed that in the radiotherapy combined with anti-PD-1 treatment group and the blank control group, the differentially expressed genes had significant changes in biological processes, cellular components, and molecular functions (Fig. 2A). In the comparisons of the radiotherapy combined with anti-PD-1 therapy group with the immunotherapy only group or radiotherapy only group, the changes in the differential gene-related enriched pathways were mainly reflected in biological processes and biological functions (Fig. 2B, 2C). Then, KEGG enrichment analysis results showed that in the comparison between radiotherapy combined with anti-PD-1 therapy and immunotherapy alone, the results were not statistically significant (adjusted p > 0.05) (Fig. 2D), while in the other comparisons, eight overlapping pathways, such as tuberculosis and toxoplasmosis, were identified, but the pathways related to a local inflammatory reaction, such as the chemokine signaling pathway and cell adhesion molecules, were significantly changed. The above results showed that different treatment modes, especially radiotherapy combined with immunotherapy, could cause changes in the enrichment of differentially expressed genes, which was mainly reflected the local inflammatory response-related pathways connected to antitumor activity.
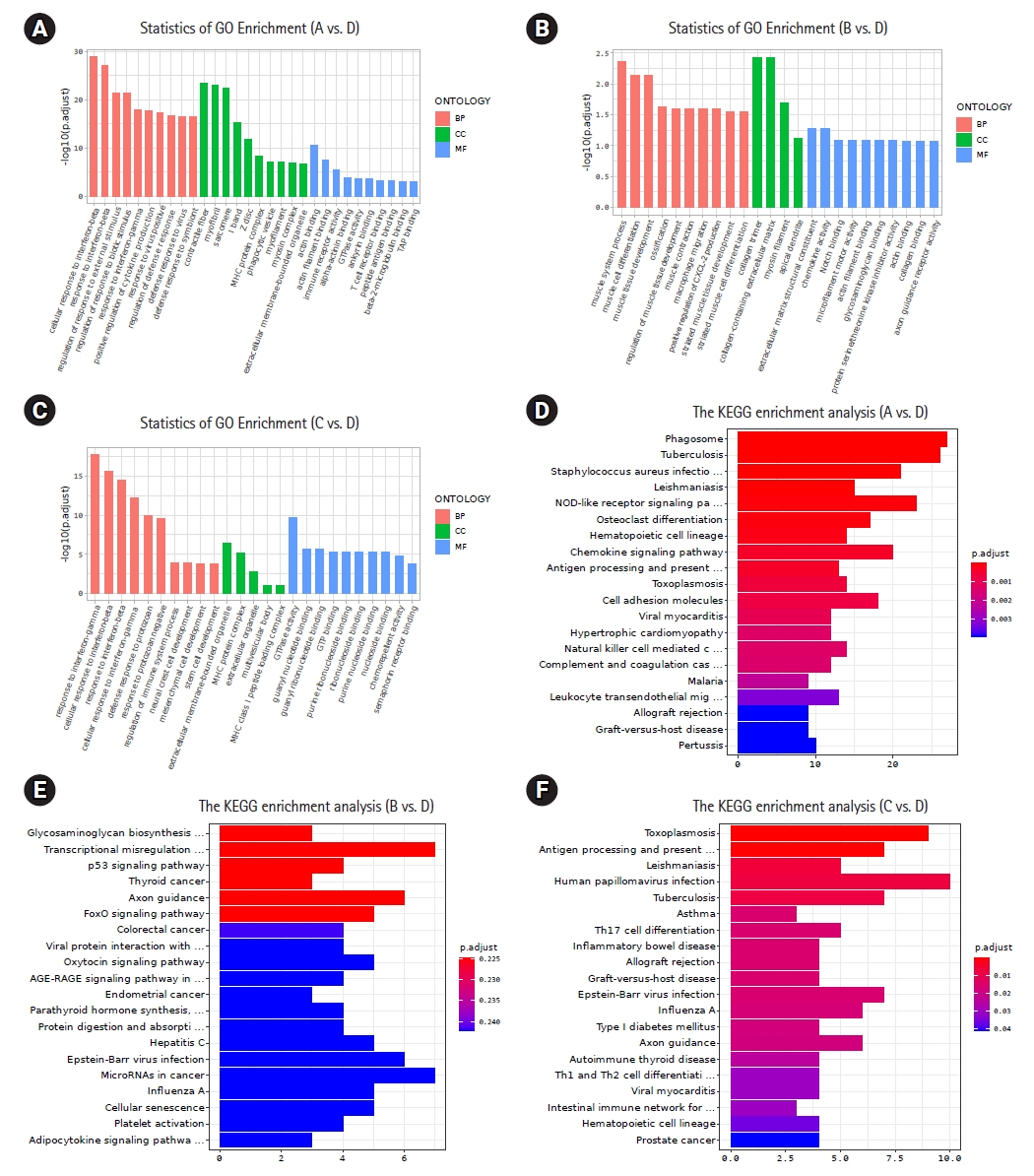
Analysis of differential genes enrichment pathways. (A–C) The Gene Ontology (GO) enrichment analysis of differential genes between the combined treatment group and other groups. Red represents biological process (BP), green represents cellular component (CC), and blue represents molecular function (MF). (D–F) The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differential genes between the combined treatment group and other groups. Adjusted p < 0.05 is statistically significant. “A” and “D” are differential genes between the combination therapy group and the control group; “B” and “E” are differential genes between the combination therapy group and the immunotherapy group; “C” and “F” are differential genes between the combination therapy group and the radiation therapy group.
3. Radiotherapy combined with anti-PD-1 therapy improved the immune microenvironment
The anti-tumor immune response depends on the proportion and function of cells in the tumor microenvironment. With the help of R language, we determined the percentages of 22 kinds of infiltrating immune cells in each sample (Fig. 3A). The degree of infiltration of each sample cell was different, but in general, the immune infiltrate was dominated by resting dendritic cells and monocytes. The T cell surface receptor PD-1 plays a key role in inhibiting the T cell response. Next, we drew a pie chart to further illustrate the differences in the percentages of immune cells in different treatment modes (Fig. 3B, 3C). In irradiated tumors, the infiltration of CD8+ T cells in both the radiotherapy group and the anti-PD-1 treatment group was increased compared with that in the blank group. In the radiotherapy combined with immunotherapy group, the infiltration of T cells was further increased. Thus, radiotherapy combined with immunotherapy was considered to improve the local infiltration of T cells. In addition, compared with other groups, the combination treatment group showed a significant change in the degree of infiltration of macrophages, monocytes, and mature dendritic cells (mDCs). In abscopal tumors, the proportions of immune cells in the control group and the immunotherapy alone group were not significantly different. Radiotherapy could increase the infiltration of monocytes in the abscopal site. The combination therapy group showed significant infiltration of mDC and T cell CD4+ compared to the radiotherapy group alone. The proportion of CD8+ T cells in the abscopal tumor in the combination treatment group was higher than that in the blank control group, but it was not significantly higher than that in the irradiated tumor. Finally, we compared the tumor purity of different sites, and the results showed that radiotherapy combined with anti-PD-1 treatment could significantly reduce the tumor purity of primary tumors, while in abscopal tumors, the tumor purity of the immunotherapy group was significantly decreased (Fig. 3D, 3E). It could be seen that different treatment modes had varying therapeutic effects on primary and distant tumors. For primary lesions, radiotherapy combined with immunotherapy was more effective, while for abscopal tumors, immunotherapy alone was more effective than combination therapy. The addition of immunotherapy on the basis of radiotherapy can significantly improve the immune microenvironment, which may be beneficial in guiding clinical treatment.
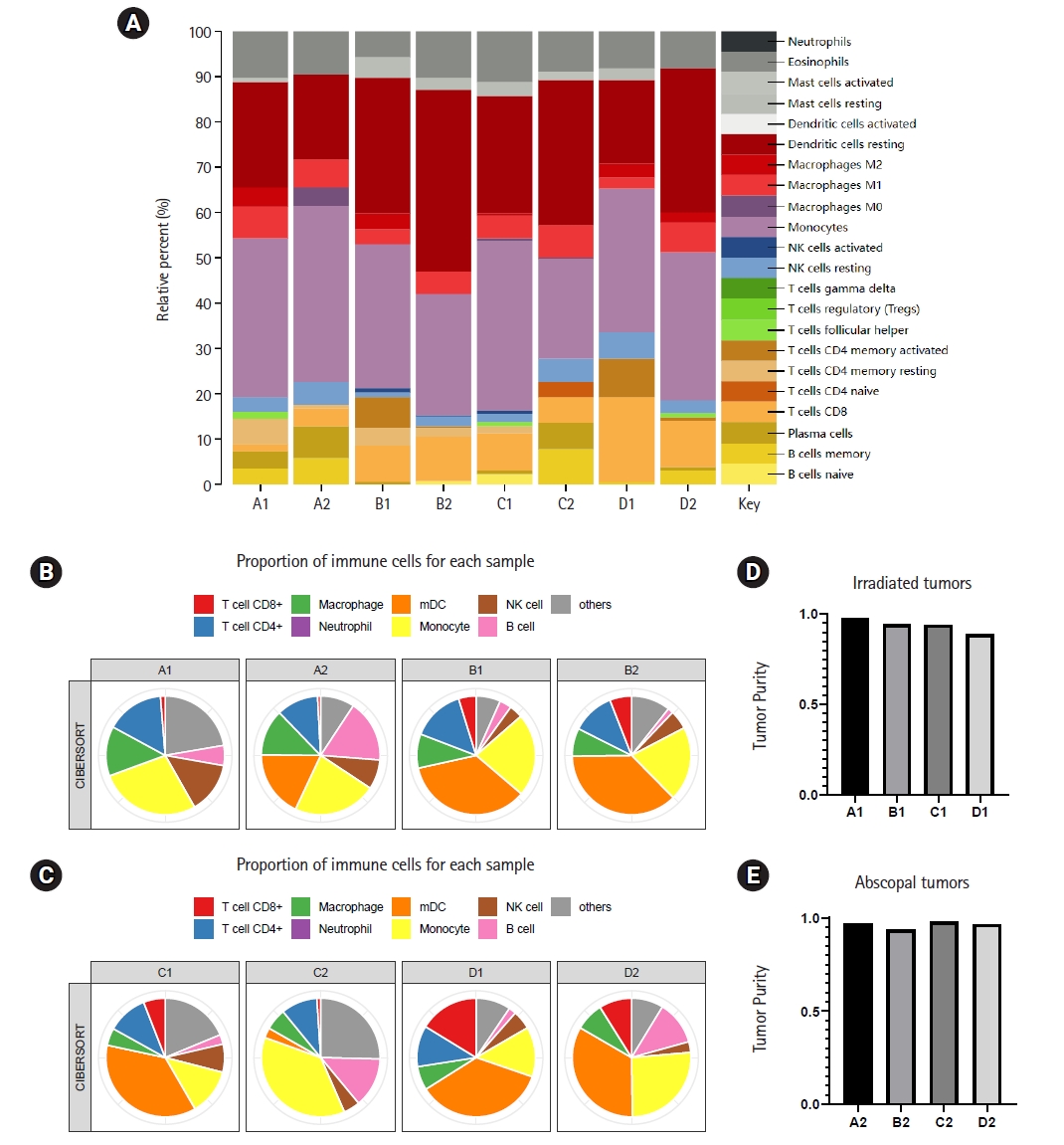
The proportion of immune cells in primary and abscopal tumors is different under different treatment modes. (A) Twenty-two kinds of immune cells infiltrate in different groups, and different colors represent different immune cells. (B, C) Pie chart shows that the proportion of main immune cells in each sample changes due to different treatment modes. (D, E) The purity of primary tumor and secondary tumor changes under different treatment modes. mDC, mature dendritic cell.
4. The abundance of immune cells in abscopal and irradiated sites was significantly changed in the radiotherapy combined with anti-PD-1 treatment group
Then, we compared the difference in the tumor immune microenvironment between the irradiated site and abscopal site. The results showed that different treatment modes caused different immune cell abundances in irradiated tumors and abscopal tumors (Fig. 4). Among the treatment modes, the radiotherapy combined with anti-PD-1 treatment group showed the greatest impact, the immunotherapy group showed the smallest impact, and the radiotherapy group was between the two. In the radiotherapy combined with anti-PD-1 treatment group, the difference in immune cell infiltration between irradiated tumors and abscopal tumors was mainly reflected in CD8+ T cells, CD4+ T cells, macrophages, monocytes, and mDCs, and the abundances of the above immune cells at the irradiated site were greater than those at the abscopal site, showing good local control.
5. Radiotherapy combined with anti-PD-1 therapy enhanced the control of irradiated tumors and abscopal tumors
To study the control of local and abscopal tumors achieved with radiotherapy combined with immunotherapy, we measured the tumor volume in the mouse abscopal effect model we established every 2 days after the tumor volume reached 150 mm3 (Supplementary Figs. S1, S2). The results showed that for the primary tumor, the tumor volume of the combination treatment group was significantly smaller than that of the control group (p < 0.01) (Fig. 5A), followed by the radiotherapy group, and immunotherapy had the weakest ability to reduce tumor volume. Among the secondary tumors, radiotherapy alone had the weakest reducing effect on tumor volume, while radiotherapy combined with immunotherapy had a significantly better tumor volume-reducing effect than the other treatments (Fig. 5B). Finally, on the 20th day of the experiment, tumors were isolated from mice (Fig. 5C). The effect of immunotherapy on the volume of secondary tumors was more obvious than that of radiotherapy alone or control treatment. The number of tumor cells subcutaneously injected to produce the secondary tumors was less than that used to establish the primary tumors, reflecting the difference in the immunosuppressive ability of immunotherapy against tumors of different volumes. Whether it was primary tumor or secondary tumors, the tumor volume in the combination therapy group was significantly reduced compared to other groups, but the change in mouse weight was not very significant (Supplementary Table S1). The mice in the immunotherapy group died on the 14th day of the experiment, the mice in the radiotherapy group died on the 16th day of the experiment, and the mice in the combination treatment group with the most obvious tumor volume reduction all lived to the end of the experiment. In summary, receiving immunotherapy alone may cause immunotoxicity and a poor prognosis. Whether considering irradiated tumors or abscopal tumors, radiotherapy combined with anti-PD-1 had the most obvious effect achieving tumor control and might have a positive impact on prognosis (Fig. 5D).

Establish a model of radiotherapy-abscopal effect in mice. (A, B) Tumor growth curve of irradiated and non-irradiated parts. N represents control group, ICB represents simple immunotherapy, RT represents simple radiotherapy. (C) Primary and secondary tumors isolated from mice were compared. From top to bottom are the control group, immunotherapy group, radiation therapy group, and combination therapy group. The missing part is the death of mice. (D) Survival percentage of each group of mice (number of surviving mice/7 * 100%). Experimental groups contained at least seven mice and are representative of two independent experiments. N: normal group; ICB: immune checkpoint blockade; RT: radiotherapy group; RT+ICB: combined therapy of RT and ICB.
Discussion and Conclusion
At present, there are various immune checkpoint blocking therapies for basic and clinical research, among which inhibitors targeting immune checkpoint cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and PD-1 molecules are at the forefront of immunotherapy [20-22]. Changes in tumor microenvironment will affect the therapeutic effect of PD-1/PD-L1 (programmed death-ligand 1) monoclonal antibody [23,24]. More and more studies have shown that radiation therapy combined with PD-1/PD-L1 monoclonal antibody can effectively expand the population benefiting from immunotherapy. There are two main mechanisms by which radiotherapy induces anti-tumor immune responses: firstly, radiotherapy kills tumor cells through radiation, significantly reducing tumor burden and alleviating tumor mediated immunosuppression [25]. The second is that radiotherapy induces immunogenic death of tumor cells and activates adaptive immunity. Radiation therapy can not only enhance local anti-tumor responses, but also produce abscopal effects by regulating the body's immune response. However, few people have studied the genetic changes caused by radiotherapy combined with immunotherapy.
Melanoma is a highly invasive tumor, and approximately 15% of patients have metastasis at the time of diagnosis [26]. Radiotherapy plays an important role in the treatment of metastatic melanoma [27]. Radiotherapy can cause differential gene expression changes, which has been widely confirmed in a variety of tumors. In our study, we found that immunotherapy could cause differential gene expression. In the radiotherapy combined with immunotherapy group, the number of differentially expressed genes was the largest, but it was not the sum of the number of differentially expressed genes resulting from radiotherapy or immunotherapy alone. According to current research, radiotherapy can activate the antitumor immune response [28], and immunotherapy has been proven to have a synergistic effect with radiation-induced immune activation [29,30]. We speculated that the interaction between radiotherapy and immunotherapy may be fundamentally caused by different genes, which further affects the changes in the antitumor pathway seen with different treatment schemes, directly leading to different antitumor effects.
Melanoma is a tumor with strong immunogenicity. Compared with other tumors, it usually shows good lymphocyte infiltration [31]. The antitumor immune response is triggered by the release of tumor antigens and proinflammatory factors, which can promote DC maturation and T-cell activation. Tumor control after radiotherapy depends largely on the T cell response [32]. Our data analysis shows that radiotherapy can slightly induce the infiltration of CD8+ T cells, while radiotherapy combined with immunotherapy can significantly increase the infiltration of it, which is consistent with current research results [14]. CD8+ T cells are an important mediator of antitumor effects and play an important role in regulating the immune response against tumor cells [33-35]. CD8+ T cells can be activated by anti-PD-1 antibodies [36,37] and then migrate to the tumor microenvironment with the help of specific cytokines [38]. In addition to the resident CD8+ T cells in the tumor microenvironment, CD8+ T lymphocytes may migrate from other immune organs or the peripheral circulation. In this paper, the infiltration of CD8+ T cells into the irradiated tumor site in the combination treatment group was significantly higher than that in the other groups, and the degree of CD8+ T cell infiltration in the abscopal tumor site in the same group was significantly different. Radiation may affect the release of cytokines in the abscopal site and thus affect the migration of immune cells. It has been reported that radiation can enhance the release of factors that recruit immunosuppressive myeloid cells [39]. Radiotherapy also induced tumor cells to release colony-stimulating factor-1, leading to the expansion of myeloid-derived suppressor cells. Radiation not only increases the number of suppressive myeloid cells in irradiated tumors but also increases the number of suppressive myeloid cells in the peripheral blood, spleen, lymph nodes, and lungs [40]. Radiotherapy combined with anti-PD-1 treatment can significantly improve T cell infiltration in the irradiated field. Although the infiltration of T cells into the abscopal tumor was not as obvious as that in the irradiated tumor, the tumor volume of each site in the combination treatment group was significantly reduced. This shows that radiotherapy can improve the immunogenicity of tumors and be used to improve the effect of immunotherapy [41,42]. At the same time, the possibility that immunotherapy has a positive impact on the efficacy of radiotherapy was not ruled out. Current research results confirm this possibility [32,43]. In the immunotherapy alone group, the number of CD8+ T cells in the secondary tumor was slightly higher than that in the irradiated tumor, while the volume of the distant tumor in this group was significantly reduced. The effect on the distant tumor depended on the presence of T cells, which is consistent with the current research conclusion [44].
X-ray irradiation can not only enhance immune efficacy but also induce direct cell death. In our study, in the radiotherapy alone group, the volume difference between the irradiated and abscopal tumors was not significant, which showed that irradiation produced an abscopal effect and the same tumor volume-reducing effect on the primary and secondary tumors. In the mouse abscopal effect model we constructed, both the irradiated and abscopal tumors in the combination treatment group significantly regressed. Radiotherapy combined with immunotherapy could not only eliminate the irradiated tumor but also enhance the abscopal effect. A reasonable combination of radiotherapy and immunotherapy can overcome immunosuppression and lead to a strong antitumor T-cell response [45,46], thus producing stronger antitumor activity. This abscopal effect has been widely confirmed in immunogenic tumors [16,47-49].
In conclusion, we explored the root causes of different therapeutic effects from different perspectives, such as gene expression and immune infiltration, providing a new idea for improving therapy in melanoma patients and inducing the abscopal effect. Combination therapy could not only improve the immune microenvironment but also induce the abscopal effect and may have a positive impact on prognosis.
Notes
Statement of Ethics
This study protocol was reviewed and approved by The Animal Care and Use Committee of Changzhou Second People's Hospital.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by National Natural Science Foundation of China (No. 82073339), Scientific Projects of Changzhou Medical Center raised by Nanjing Medical University (No. CMCB202201), and partly by Changzhou Sci&Tech Program (No. CJ20220224, CJ20220092).
Author Contributions
Conceptualization, MHW, JDL. Investigation and methodology, YFZ, XL, NL. Resources, ZQS, AMZ. Writing of the original draft, XL. Formal analysis, YFZ. All authors had finally approved the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2023.00185.
(A) Mice receiving a single dose of 20 Gy of 6 MeV electron rays from the Elekta Infinity linear accelerator. The irradiation field was 20 cm × 5 cm and the SSD was 100 cm. (B) Irradiate five mice side by side each time.
Tumor volume measurement.
Dynamic monitoring of tumor volume and mouse body weight

