Introduction
New technologies in radiotherapy (RT) have been developed to overcome the low tolerance of normal tissue and achieve a high therapeutic ratio. For example, image-guided RT has been used to identify the exact tumor location and the relationship between the tumor and normal organs. Intensity-modulated RT was developed to deliver high doses of radiation precisely to the tumor location by using a stiff dose gradient. Despite the use of these technologically advanced methods, tumor responses are limited in cases of hypoxic tumors. Hypoxia is a key regulatory factor in tumor growth [1] and plays a crucial role in RT resistance [2]. Furthermore, RT can result in cycling of hypoxia in tumor regions [3]. After RT, the lack of oxygen reduces the production of reactive oxygen species (ROS), and ultimately prevents irreparable DNA damage from occurring in tumor cells, thereby preventing tumor cell death [4]. Hypoxia also leads to the up-regulation of hypoxia-inducible factor 1α (HIF-1α), which independently promotes radioresistance [5].
One of measures to overcome the limitations of RT is the use of nanoparticles in RT. Nanoparticles are utilized for various purposes in antitumor treatments, such as drug delivery [6,7], diagnosis [8,9], gene therapy [10], and RT [11]. When nanoparticles are combined with RT, absorbed radiation dose increases. In particular, the use of gold nanoparticle (GNPs) to enhance RT efficacy is well established [12,13]. GNPs can enhance RT efficacy owing to their high K-edge [14]. Studies have explained the enhancement of RT using GNPs in medical physics [15,16]. One of the reasons for the enhancement of RT efficacy when used in combination with GNPs is ROS production when GNPs are subjected to X-ray irradiation. This ROS generation differs depending on the composition, size, and potential of GNPs [17].
We hypothesized that increased ROS production due to GNPs combined with RT overcomes the limited effect of RT under hypoxic conditions. This study was aimed at investigating the enhancement of RT efficacy using GNPs and evaluating the potential for increasing RT efficacy under hypoxic conditions.
Materials and Methods
1. Reagents
HIF-1α antibody was purchased from Abcam (Cambridge, UK), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (St. Louis, MO, USA).
2. GNP synthesis
The GNPs used in this study consisted of a silica core with a gold shell. Silica nanoparticles (SNPs) were functionalized with 3-aminopropyl triethoxysilane to help attachment of gold seeds (1–2 nm). Gold seeds were deposited onto the SNPs. Further gold coverage of the SNPs was accomplished by the reduction of gold hydroxide by hydroxylamine hydrochloride on the SNP surface (Fig. 1A) [18].
3. Cell culture
Mouse CT26 colon cancer cells were used for all experiments. The cells were cultured in RPMI 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics and maintained at 37°C in a 5% CO2 incubator. They were subcultured every 3–4 days to maintain exponential growth.
4. MTT assay
Mouse CT26 colon cancer cells (4 × 103 cells/well) were plated onto 96-well plates. After 24 hours, the cells were incubated with 2 μg/mL GNP for 24 hours at 37°C in a 5% CO2 incubator. After incubation, the cells were washed with phosphate buffered saline (PBS) to remove excess GNPs. The effect of GNPs on cell viability was evaluated using the MTT assay at 24 hours after GNP exposure.
5. Apoptosis assay
For in vitro apoptosis assay, cells were treated GNP with 2 μg/mL for 24 hours, which was washed with new media followed by irradiation with 8 Gy. After incubation for 48 hours, cells were stained with FITC-annexin V (BD Biosciences, San Jose, CA, USA) and propidium iodide (PI; BD Biosciences) for 15 minutes. Apoptotic cells were evaluated by flow cytometry analyses using FACSVerse flow cytometer (BD Biosciences).
6. Effect of the combination of GNPs and RT on cell growth under hypoxic conditions
To investigate the synergistic effect of GNPs and RT on cell growth inhibition under hypoxic conditions in comparison with that of RT alone, 4 × 103 cells/well were incubated with 2 μg/mL GNP for 24 hours at 37°C in a 0.1% O2, 5% CO2, and balanced N2, respectively. After incubation, the cells were washed with PBS to remove excess GNPs followed by 8 Gy irradiation. A single fraction of 8 Gy was delivered to the tumor cells by using a X-RAD 320 irradiator (Precision X-Ray, North Branford, CT, USA) and cell viability was evaluated using the MTT assay.
7. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
The TUNEL assay was performed according to the manufacturer’s instructions (Promega Co., Madison, WI, USA). Briefly, after deparaffinization and rehydration, tissue sections were incubated with proteinase K (20 μg/mL) for 15 minutes at 24°C and washed in PBS. The slides were incubated with a mixture of TdT enzyme and biotinylated nucleotide for 60 minutes at 37°C. After being washed in PBS, the slides were incubated with streptavidin-horseradish peroxidase (HRP) solution for 30 minutes at 24°C. To detect TUNEL-positive signals, the slides were incubated with a mixture of 3,3’-diaminobenzidine (DAB) substrate, chromogen, and hydrogen peroxide. The slides were then examined and the images were recorded using a microscope (Olympus BX53F; Olympus, Tokyo, Japan). Dark brown staining represented a positive reaction. Positive reactions showed under the microscope, and stained cells were quantified by stereological analysis using Image-J software (https://imagej.nih.gov/ij/).
8. ROS evaluation
CT26 cells were cultured in a 6-well plate for 24 hours followed by 6 Gy irradiation. After incubation for 2 hours, the cells were treated with a fluorogenic probe, as an indicator, for ROS detection and measurements were performed using a fluorescence microscope (Zeiss, Jena, Germany). Dichlorodihydrofluorescein, supplied as a diacetate ester (DCFH DA), is readily oxidized to the highly fluorescent dichlorofluorescein (DCF) after enzymatic or base-catalyzed cleavage of the diacetate groups; 10 mM N-acetyl-L-cysteine (NAC) was used as a ROS scavenger.
9. Tumor growth inhibition (TGI)
The CT26 mouse colon cancer model was developed to investigate the synergistic effect of GNP in combination with RT on tumor growth. We injected 106 CT26 mouse colon cancer cells into the thigh of BALB/c mice subcutaneously. When tumor diameter reached 6 mm or 12 mm, GNPs (100 μg Au) were injected into tumors; thereafter, the tumors were irradiated with 10 Gy in a single fraction by using X-RAD 320 irradiator (Precision X-Ray). The mice were placed at a distance of 69 cm from the radiation source and treated at a dose rate of 150 cGy/min with 300 kVp X-rays, using 12.5 mA and a X-ray beam filter consisting of 2.0 mm Al. Tumor volume was calculated using the formula 0.5 × ab2, where a is the long axis and b is the short axis of two orthogonal diameters. The mice were divided into the following 4 groups (A, control; B, GNPs; C, RT; D, GNPs + RT). Tumor growth delay was observed for 18 days.
10. Immunofluorescence staining for HIF-1α detection
Twenty four hours after irradiation, mice were sacrificed. Tumors were fixed in 4% paraformaldehyde and embedded in paraffin. The blocks were cut into 5-μm-thick sections. Antigen retrieval was accomplished at 37°C with protease K solution. For immunofluorescence staining, deparaffinized sections were blocked with 10% normal horse serum for 1 hour and then incubated with primary antibodies against HIF-1α for overnight at 4°C (Abcam). After washing with PBS, the samples were incubated for 1 hour with a PE conjugated secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA). Reactions showed under the fluorescence microscope, and HIF-1α stained cells were quantified using Image-J software.
Results
1. GNP characterization
The GNPs were imaged using transmission electron microscopy (Fig. 1B). The formation of GNPs was first confirmed by the color of the nanoparticle-containing solution. As further gold coverage was achieved, the color of the GNP-containing solution changed from brown to blue-green (Fig. 1C), and the absorbance peak of the solution widened and red-shifted (Fig. 1D). After addition of 0.250 mM gold hydroxide, a fairly thick GNP was obtained. The mean diameter of the GNPs was 180 nm. The resultant GNPs exhibited a small absorbance peak at 540 nm [18]. The cytotoxicity of GNPs was evaluated using the MTT assay. GNPs did not affect cell viability until 100 µg/mL (Fig. 1E).
2. GNPs enhanced the radiosensitivity of tumor cells
To investigate synergistic effect of GNPs used in combination with RT on TGI, CT26 cells were treated with GNPs in the absence or presence of radiation. After administration of 2, 8 Gy in a single fraction to CT26 tumor cells, a dose-response relationship was observed at various concentrations of GNPs (Fig. 2A). Tumor cells treated with 8 Gy showed less survival than that shown by the cells treated with 2 Gy. As the concentration of the GNPs increased, cell viability slightly decreased.
GNPs combined with RT increased apoptosis significantly (Fig. 2B). In comparison with cells treated with RT alone, those treated with GNPs + RT showed significantly greater apoptosis.
3. Synergistic effect of GNPs in combination with RT on TGI in hypoxia
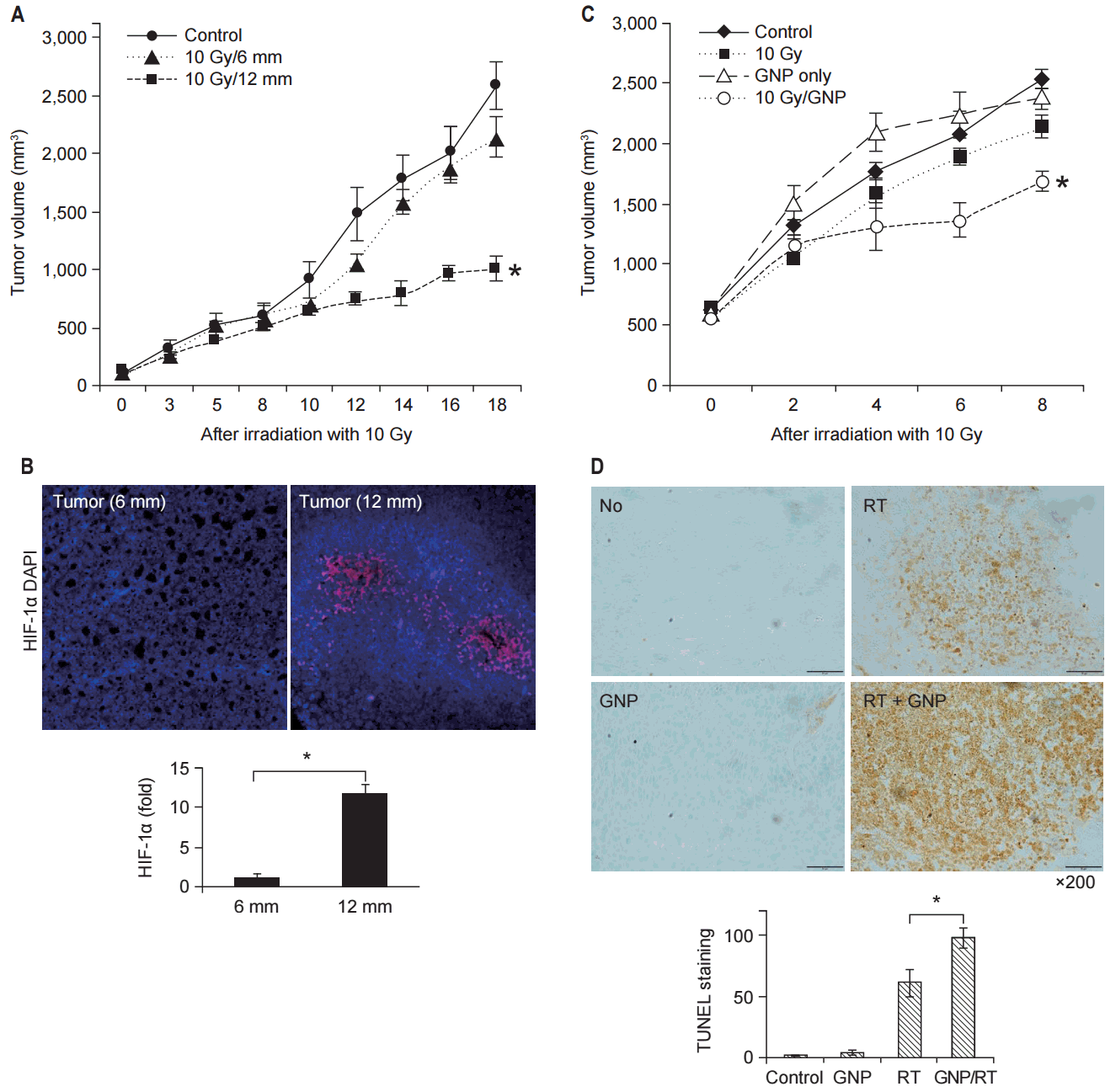
To evaluate the effect of hypoxia on TGI, tumors with different diameters after treatment with 10 Gy in a single fraction were compared (Fig. 3A). While the tumor with a diameter of 6 mm showed significant TGI after irradiation, the tumor with a diameter of 12 mm did not show significant TGI. Immunohistochemical staining for HIF-1α showed that tumor with the diameter of 12 mm had more hypoxic tumor cells (Fig. 3B).
To evaluate the synergistic effect of GNPs in combination with RT under hypoxic conditions, tumor volumes of the mice in the 4 groups (A, B, C, and D) were compared (Fig. 3C). Group D (GNPs + RT) showed better TGI than that shown by group C (RT). During the follow-up period, groups A-C showed no difference in tumor volume. Apoptosis after irradiation was investigated using TUNEL staining. The number of TUNEL-positive cells increased in group D compared to those in groups A-C (Fig. 3D).
4. GNP induced ROS-dependent apoptosis
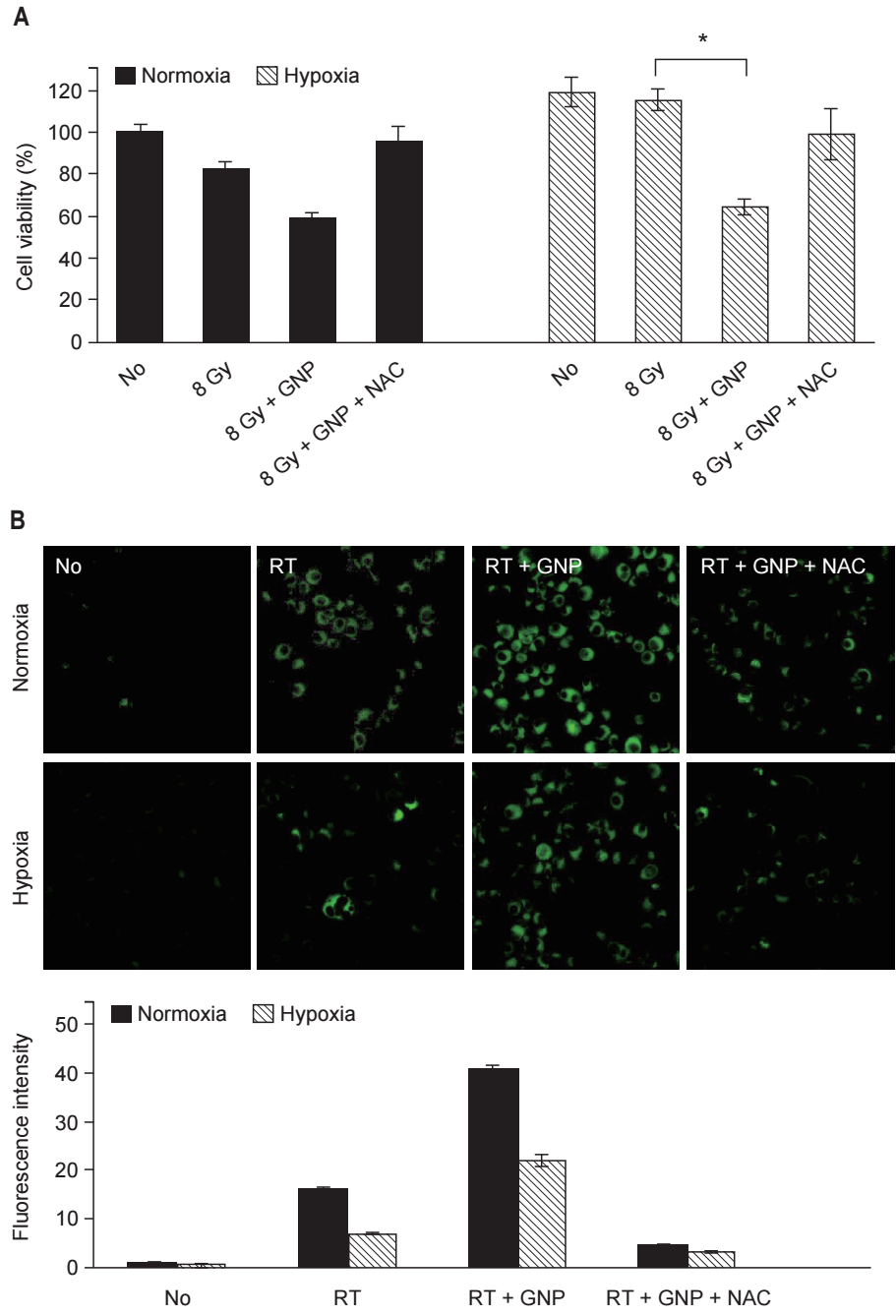
To investigate the mechanism of the synergistic effect of GNPs combined with RT, cell viability was compared using the MTT assay (Fig. 4A). Under normoxic conditions, GNPs combined with RT showed an enhanced antitumor effect on tumor cells. Tumor cells treated with GNPs + RT had lower survival than those treated with RT alone. Meanwhile, the improved antitumor effect diminished when NAC added. Under hypoxic conditions, the enhancement of the effect of RT due to GNPs was more notable. The effect of RT on cell viability under hypoxic conditions was slight. GNPs combined with RT showed a synergistic effect and the survival of the cells treated with GNPs + RT was significantly lower than that of the cells treated with RT alone.
ROS generation was observed using a fluorogenic probe (Fig. 4B). Under normoxic conditions, ROS generation was detected in cells treated with RT. The fluorescence intensity increased significantly in the cells treated with GNPs + RT. Fluorescence intensity decreased upon addition of NAC, and the same patterns were observed under hypoxic conditions. The antitumor effect of GNPs + RT was correlated with ROS generation. When ROS generation increased, the survival of cells decreased. When ROS was removed using NAC, the survival of cells recovered.
Discussion and Conclusion
Oxygen is well-established radiosensitizer. Free radicals generated after irradiation break the DNA double strand and induce biological damage in tumors. Oxygen fixes DNA damage, resulting in enhancement of RT efficacy [19]. Therefore, the oxygen effect is limited under hypoxic conditions.
Tumor cells often are under hypoxic conditions. In particular, in large tumors, some tumor cells are located far from blood vessels. Tumor blood vessels are immature, and therefore, temporary obstruction occurs often. Hypoxic conditions affects both the DNA damage repair pathway and cell survival signaling pathway [20]. HIF-1α becomes activated in hypoxic conditions, and results in increased expression of genes related to angiogenesis, invasion and metastasis of tumor cells [3].
We observed that hypoxic tumor cells had an impaired response to RT. GNPs combined with RT enhanced the antitumor effect in hypoxic tumor cells. After irradiation, the survival of hypoxic tumor cells was higher than that of normoxic tumor cells. Addition of GNPs decreased tumor cell viability significantly. The tumor growth was impaired in both small tumor and large tumors after administration of 10 Gy in a single fraction. However, large tumors, which had more hypoxic tumor cells, showed a poor response. When we evaluated tumor growth after the administration of 10 Gy in a single fraction, the tumors treated with GNPs + RT showed a higher response than that shown by the tumors treated with RT alone. With regard to tumor cell death, the cells treated with GNPs + RT and NAC showed a higher survival rate than that shown by the cells that were not treated with NAC. Apoptosis due to ROS was one of the main mechanisms of cell death.
In clinical practice, stereotactic body radiotherapy (SBRT) is used to overcome the radioresistance of hypoxic tumors [21]. SBRT delivers an ablative radiation dose to the tumor area in 1–5 fractions. However, stereotactic radiosurgery and SBRT with the current doses cannot directly kill all the hypoxic cells in the tumor. GNPs when combined with RT enhance RT efficacy. Gold has a high atomic number. It is advantageous to interact with KeV energy radiation (photoelectric effect). Due to the photoelectric effect, short-range photo-Auger electrons emitted from the GNPs boost the tumor area while sparing the adjacent normal tissue.
Some studies pointed out the discrepancy between predicted dose enhancement via a photoelectric effect and the radiobiological response of GNPs combined with RT [22,23]. Butterworth and colleagues focused on ROS. GNPs combined with RT elicit tumor cell responses mediated through oxidative stress. They suggested that oxidative stress via ROS is one of the main mechanisms of tumor response. GNPs contribute to increased ROS production when a tumor is irradiated. Misawa and colleagues studied the factors that affected ROS generation in the presence of GNPs [24]. The Auger electron and fluorescent X-ray, which were the resultants of interaction of ionizing radiation with GNPs, cause secondary water radiolysis. In addition to primary water radiolysis, secondary water radiolysis can produce additional ROS. Our study also showed increased ROS generation upon using GNPs. We used a cell-permeable fluorogenic probe to detect the presence of ROS. Tumor cells treated with GNPs + RT showed increased ROS generation.
GNPs in combination with RT induce decreased clonogenic cell survival, increased apoptosis, and DNA damage [23], which are related to oxidative stress. Therefore, GNPs combined with RT may synergistically cause radiochemical damage via ROS [24]. In the present study, tumor cells treated with GNPs + RT exhibited increased apoptosis. It was correlated with ROS generation. In hypoxic conditions, GNPs enhanced RT efficacy. The viability of tumor cells decreased significantly when treated with GNPs + RT. However, the effect was diminished when a ROS scavenger was added.
GNPs combined with RT may have boost effect to tumor area. Berbeco et al. [16] reported a boosting dose for tumor endothelial cells using megavoltage X-rays. They showed local dose enhancement for the tumor microvasculature by using linear accelerator X-ray. They found that GNPs combined with external-beam RT could deliver a prescribed dose to tumor cells and boost dose to the tumor microvasculature concomitantly. Tumors with disrupted microvasculature are deficient in nutrients and oxygen, which leads to necrosis. Rapid intratumoral necrosis results in remnant viable tumor cells around the margin of the necrotic area. The remnant viable tumor cells are close to normal tissue vasculature, and could be re-oxygenated, making them good targets for RT [25]. In our study, tumors treated with GNPs + RT showed better responses than those treated with RT alone during the follow-up period. After administration of a single fraction of radiation, remnant viable tumor cells close to the normal vasculature might be re-oxygenated, and the oxygen effect might induce increased tumor cell death.
This study was insufficient to gather robust evidence for the enhancement of RT efficacy by GNPs to overcome hypoxia. GNPs were administered by intratumoral injection, and we could not be sure that they were distributed evenly in the tumor. The absorbed dose was not measurable. It could be odd that tumors with 12 mm diameter were defined as hypoxic tumors based on HIF-1α immunohistochemistry staining. Tumors in different size may have different characteristics. Furthermore, hypoxia is not the only factor that can influence on radiation sensitivity. In future studies, we should follow a way to make hypoxic tumor with similar size as a previous work [26].
Finally, in this study, GNPs combined with RT exhibited a favorable response in hypoxic tumors, which were resistant to RT. RT administered with high-tech therapeutic machinery alone has not shown a favorable effect in hypoxic tumors and tolerability in normal tissues, simultaneously. However, GNPs combined with RT enhanced ROS generation in hypoxic tumors, leading to tumor cell apoptosis. Thus, GNPs combined with RT may have potential for enhance the efficacy of RT. We expect further studies on clinical application of GNPs combined with RT.