 |
 |
AbstractPurposeTo investigate interobserver variation in target volume delineations for prostate cancer salvage radiotherapy using planning computed tomography (CT) versus combined planning CT and magnetic resonance imaging (MRI).
Materials and MethodsTen radiation oncologists independently delineated a target volume on the planning CT scans of five cases with different pathological status after radical prostatectomy. Two weeks later, this was repeated with the addition of planning MRI. The volumes obtained with CT only and combined CT and MRI were compared, and the effect of the addition of planning MRI on interobserver variability was assessed.
ResultsThere were large differences in clinical target volume (CTV) delineated by each observer, regardless of the addition of planning MRI (9.44ŌĆō139.27 cm3 in CT only and 7.77ŌĆō122.83 cm3 in CT plus MRI) and no significant differences in the mean and standard deviation of CTV. However, there were decreases in mean volume and standard deviation as a result of using the planning MRI.
IntroductionSalvage radiotherapy (RT) to the prostatic fossa is an effective therapy in patients with biochemical or local recurrence after radical prostatectomy (RP). The American Society for Radiation Oncology (ASTRO) and the American Urological Association (AUA) guidelines recommended salvage RT for treatment of biochemical or local recurrence without evidence of distant metastasis [1]. Several randomized trials have included a subgroup with detectable prostate-specific antigen (PSA) levels after RP. RT after RP significantly reduced local failure and distant metastasis and improved overall survival in the Southwest Oncology Group (SWOG) 8794 [2,3] and biochemical failure in the European Organisation for Research and Treatment of Cancer (EORTC) 22911 [4] among patients with postoperative high-risk factors or a detectable PSA level. Two observational studies have reported the outcomes of salvage RT. At a median of 11.5 years, salvage RT after RP significantly reduced the local recurrence risk by almost 90% and systemic progression by 75% [5] and was associated with a three-fold increase in prostate cancer-specific survival relative to those who received no salvage treatment [6].
Although salvage RT is the only potentially curative treatment option for recurrent prostate cancer patients after RP, the optimal target volume of salvage RT remains controversial. Several guidelines have been published for clinical target volume (CTV) delineation in postoperative RT [7-10]; however, there is no universally accepted method, and substantial variation exists.
In the postoperative setting, anatomic changes that occur following RP may significantly alter the target definition compared with definitive RT for localized prostate cancer patients. The absence of a visible target introduces a potentially significant interobserver bias in defining the prostatic fossa CTV.
Magnetic resonance imaging (MRI) provides greater resolution of soft tissues compared with computed tomography (CT), and it allows more precise delineation of target volume and normal tissues [11,12]. Therefore, MRI is commonly used in the planning of definitive RT for prostate cancer. Several studies have reported that addition of MRI to planning CT decreases target volume and reduces variation in prostate delineation [13,14]. Also, MRI has the potential to demonstrate the site of local recurrence after RP [7-9] and can be used for CTV delineation in salvage RT.
In this study, we investigated interobserver variation in target volume delineation for salvage RT in recurrent prostate cancer patients treated with radical prostatectomy, and we compared the effect of addition of MRI in determination of CTV.
Materials and Methods1. Case and observer characteristicsPlanning CT scans of five sampled cases referred for salvage RT to the prostatic fossa because of continuous PSA elevation after RP were used for this target delineation study. Each case involved diagnostic prostate MRI before RP and planning MRI before salvage RT. The clinical and surgical information of the cases is shown in Table 1.
Ten radiation oncologists from 10 different institutions participated in the study and were asked to contour the target volume on each of the five CT scans. Their institutionsŌĆÖ practice patterns for salvage RT are described in Table 2.
2. Planning CT and MRI acquisitionThe planning CT and MRI scans were performed in a reproducible supine position with endorectal ballooning at same day. All patients voided their bladders immediately before scanning. Treatment planning CT scans of the pelvis were obtained in 2.5-mm slice thicknesses at 2.5-mm intervals using a LightSpeed RT16 scanner (GE Healthcare, St. Giles, UK). The MRI scans were performed with 3.0T systems (Achieva 3T, Philips Medical System, Best, Netherlands) using a 6-channel phased-array coil. Both T2-weighted images (T2WIs) and diffusion-weighted images (DWIs) were included in all of the MRI scans. The T2WIs were acquired in the three orthogonal planes (axial, sagittal, and coronal). The following T2WI scan parameters were used for this study: repetition time (TR)/echo time (TE), 2,600-4,200 ms/80ŌĆō100 ms; slice thickness, 3 mm; interslice gap, 0.3ŌĆō0.1 mm; matrix, 512├Ś304; field-of-view (FOV), 15ŌĆō20 cm; number of signals acquired, 3; and sensitivity encoding (SENSE) factor, 2. The DWIs were acquired in the axial plane using the single-shot echo-planar imaging technique. The scanning parameters were as follows: TR/TE, 2,740ŌĆō2,750 ms/83ŌĆō85 ms; slice thickness, 3 mm; interslice gap, 1 mm; matrix, 112 ├Ś 110; FOV, 20 cm; SENSE factor, 2; and the number of signals averaged (NSA), 3.
3. Target volume delineationTen observers received the clinical information and pathological status of five cases in order to define CTV (Table 1). The planning CT and diagnostic MRI (T2WI and DWI) scans were offered to each observer using an online storage system and were transferred to each individual planning system. On every CT slice, the first contouring of CTV was performed under institutional practice guideline whether or not diagnostic MRI was performed. After an interval of 2 weeks, the planning MRI scans (T2WI with endorectal ballooning and DWI) of each case were offered and superimposed on the CT image. The second contouring of CTV was drawn on superimposed CT and axial MRI slices of each scan. All CTVs included only the prostate fossa (pelvic lymph node area was excluded).
4. Analysis of interobserver variationAfter completion of contouring, planning CT images with two different CTVs were analyzed using the Pinnacle3 planning system 9.2 (Philips Medical Systems, Fitchburg, WI, USA). Data on each delineated target volume both on planning CT only (CTV1) and on CT with planning MRI (CTV2) were imported from the RT planning system. For each case, the average volume and standard deviation (SD) of the ten observer measurements were calculated. Subsequently, the mean of these averages and their SDs were calculated for both CTV1 and CTV2. Comparisons between CTV1 and CTV2 derived values (mean or SD) were represented in percentage decrease, defined as (xCTV2ŌĆōxCTV1)/xCTV1; positive values indicated that the CTV2 value was larger than the corresponding CTV1 value.
Statistical analysis was executed using SAS ver. 9.3 (SAS Institute, Cary, NC, USA). The Wilcoxon signed-rank test was used to compare measurements of target volume. A linear mixed model was used to identify the factors that influenced target volume delineation. All p-values were two-sided, and those less than 0.05 were regarded as statistically significant.
Results1. Interobserver variations in CTV1 and CTV2There were large differences in CTV delineated by each observer, regardless of the addition of planning MRI. Table 3 shows the CTVs determined by CTV1 and CTV2 for each case. Between the observers, 7- to 15-fold differences existed.
2. Target volume change using planning MRIThere were no significant differences in mean and SD between CTV1 and CTV2 (50.6 ┬▒ 31.6 cm3 in CTV1 and 49.7 ┬▒ 30.6 cm3 in CTV2; p = 0.1875). The mean CTV2 of the observers decreased compared with CTV1 for all except case #1. The SD of CTV2 was also decreased compared with CTV1 for all except case #4 (Fig. 1A). The percentage decrease in the CTVs is demonstrated in Fig. 1B. The observers tended to report a decreased mean volume and SD as a result of using the planning MRI.
3. CTV1 and CTV2 difference according to observersThe target volumes were also analyzed by each observer. Fig. 2 shows mean CTVs and standard errors of each observer. Observer 10 reported the largest volume (94.7 ┬▒ 27.2 cm3 in CTV1 and 95.1 ┬▒ 24.6 cm3 in CTV2), and observer 1 reported the smallest volume (11.3 ┬▒ 1.5 cm3 in CTV1 and 10.6 ┬▒ 2.4 cm3 in CTV2). Six observers delineated the decreased CTV (CTV2) using planning MRI.
Discussion and ConclusionIn adjuvant or salvage RT after RP, there is no optimal consensus for CTV delineation. Several guidelines have been published based on the pattern of local failure. Malone et al. [15] compared four of the guidelines [7-10] and reported that the recommended CTV was significantly different among the guidelines. The CTVs proposed by the EORTC, the Faculty of Radiation Oncology Genito-Urinary Group (FROGG), the Radiation Therapy Oncology Group (RTOG), and the Princess Margaret Hospital (PMH) differ based on the recommended target border, especially in the cranial direction. The superior boundary of the CTV was defined as follows: by the bladder neck in the EORTC guideline; as encompassing the entire seminal vesicle bed and distal portion of the vas deferens in the FROGG guideline; as the level of the cut end of the vas deferens or 3ŌĆō4 cm above the top of the symphysis in the RTOG guideline; and as the superior surgical clips or 5 mm above the vas deferens in the PMH guideline. The volumes of the four guidelines were 60 ┬▒ 17 cm3, 88 ┬▒ 16 cm3, 102 ┬▒ 24 cm3, and 104 ┬▒ 25 cm3, respectively.
Recently, we analyzed the recurrent tumor location in 113 prostate cancer patients using MRI and suggested an optimal CTV, which included 97% of suspected tumor recurrences. Using the inferior border of the pubic symphysis as a point of reference, the inferior border of the CTV was 8 mm below and the superior border extended 30 mm above. We suggested that it might be unnecessary to routinely include the seminal vesicle bed or the surgical clips within the CTV for all patients. With this guideline, the mean CTV in our practice was 15 ┬▒ 5 cm3, remarkably smaller than those of the other guidelines [16].
In this study, there were large differences in CTV, regardless of planning MRI fusion between observers. These differences were due to the differing CTV delineation guidelines among the different institutions. Our results indicate that CTV delineation by fusion of planning CT and planning MRI might reduce clinical target volume.
The organ-discriminating power on CT images is much lower than that on MR images. CT can be used to discriminate various tissues based solely on differences in attenuation coefficients [10]. Since the prostate, rectal and bladder wall, pelvic floor muscles, and penile bulb have similar attenuation coefficients, they cannot readily be discriminated on CT images, resulting in potentially significant inaccuracies. Conversely, MRI can be used to demonstrate and characterize soft tissues by providing superb soft-tissue contrast on T2WIs. MRI therefore shows much more detail on the prostatic margins in any direction, leading to more accurate delineations. Compared with MRI, CT images overestimate prostate volume by 35% [14]. Villeirs et al. [13] reported that using CT plus MRI compared to CT alone resulted in significant decreases of 6.54%, 5.21%, and 10.47% in mean CTV of prostate and seminal vesicle volumes, and the SDs were significantly decreased by 63.06%, 62.65%, and 44.83%, respectively. These results indicate that the addition of MRI to CT decreases the interobserver target volume delineation variation in definitive RT for prostate cancer. Therefore, this greater resolution of soft tissues through the use of MRI could help the delineation of CTV for salvage RT through the anatomic precision of prostatic fossa.
Despite a substantial difference between CTVs delineated by each observer, a trend of volume reduction was observed in the mean CTV and SD when planning MRI was added. When we considered salvage RT procedures of 10 institutions, the observers using endorectal ballooning and bladder filling in clinical practice affected the CTV and had a tendency of decreasing target volume with MRI. Anatomic accuracy of prostatic fossa using planning MRI might be one explanation for the results.
In conclusion, we show that interobserver variation in target volume delineation for salvage RT is substantial. The combination of planning MRI with CT tends to decrease the target volume and its variation. However, the role of MRI in salvage RT target delineation was insufficient, and additional studies are needed. Furthermore, to reduce interobserver variation, a consensus meeting for optimal target volume after RP is needed among physicians.
Fig.┬Ā1.(A) Mean volume with standard error and (B) percentage decrease of clinical target volume (CTV) in each case. CTV1, delineated by planning computed tomography only; CTV2, delineated by planning computed tomography fusion with magnetic resonance image. 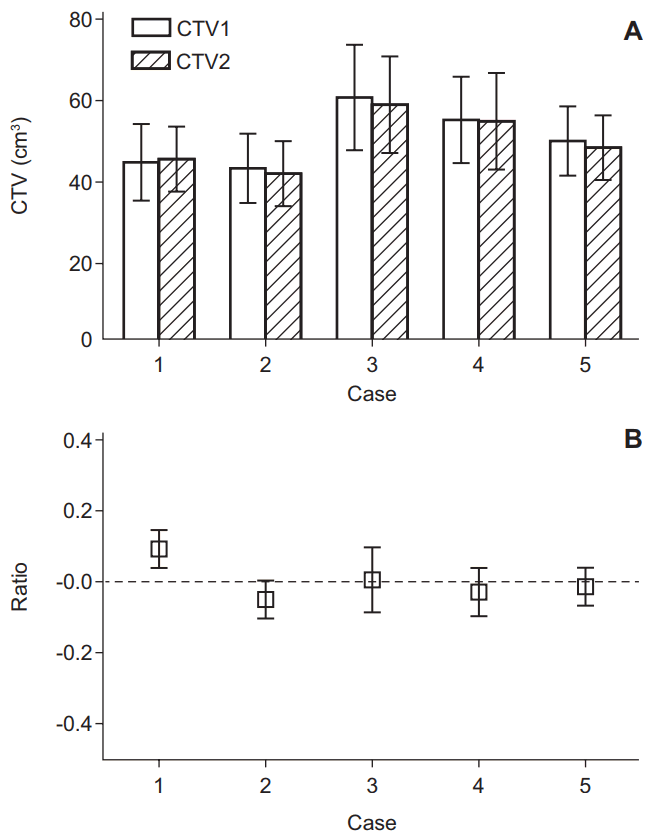
Fig.┬Ā2.Mean volume with standard error of all 5 cases in each observer. CTV, clinical target volume; CTV1, delineated by planning computed tomography only; CTV2, delineated by planning computed tomography fusion with magnetic resonance image. 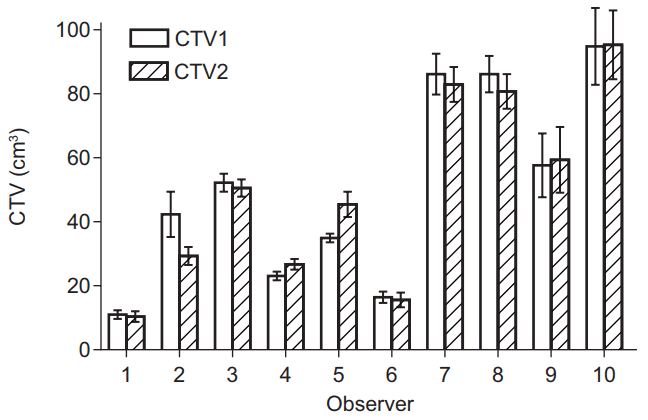
Table┬Ā1.Case characteristics
Table┬Ā2.InstitutionsŌĆÖ radiotherapy practice Table┬Ā3.Interobserver variations of CTV according to acquired planning images References1. Valicenti RK, Thompson I Jr, Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys 2013;86:822ŌĆō8.
2. Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol 2007;25:2225ŌĆō9.
3. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009;181:956ŌĆō62.
4. Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomized controlled trial (EORTC trial 22911). Lancet 2005;366:572ŌĆō8.
5. Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, Blute ML. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol 2009;182:2708ŌĆō14.
6. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760ŌĆō9.
7. Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol 2007;84:121ŌĆō7.
8. Sidhom MA, Kneebone AB, Lehman M, et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol 2008;88:10ŌĆō9.
9. Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys 2007;69:1090ŌĆō9.
10. Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361ŌĆō8.
11. Roach M 3rd, Faillace-Akazawa P, Malfatti C, Holland J, Hricak H. Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 1996;35:1011ŌĆō8.
12. Villeirs GM, L Verstraete K, De Neve WJ, De Meerleer GO. Magnetic resonance imaging anatomy of the prostate and periprostatic area: a guide for radiotherapists. Radiother Oncol 2005;76:99ŌĆō106.
13. Villeirs GM, Van Vaerenbergh K, Vakaet L, et al. Interobserver delineation variation using CT versus combined CT + MRI in intensity-modulated radiotherapy for prostate cancer. Strahlenther Onkol 2005;181:424ŌĆō30.
14. Hentschel B, Oehler W, Strauss D, Ulrich A, Malich A. Definition of the CTV prostate in CT and MRI by using CT-MRI image fusion in IMRT planning for prostate cancer. Strahlenther Onkol 2011;187:183ŌĆō90.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |