 |
 |
AbstractPurposeThe standard treatment of non-Hodgkin lymphoma (NHL) comprises combined modality treatment, radiotherapy (RT), and chemotherapy with rituximab which has significantly improved both disease-free survival (DFS) and overall survival (OS). However, there is no uniformity in radiation dose usage in these patients. In this retrospective study, we compared lower radiation dose with higher in patients with aggressive NHL.
Materials and MethodsFrom 2007 to 2017, treatment records of all high-grade NHL or diffuse large B-cell lymphoma and non-central nervous system NHL were included. We compared response rates, OS and DFS of patients who received ≤30 Gy RT to those with >30 Gy. Univariate and multivariate analyses were done to determine factors affecting prognosis, i.e., age, sex, stage, International Prognostic Index (IPI), adding rituximab, and radiation dose.
ResultsA total of 184 NHL patients treated with combined modality or radiation alone having complete follow-up details were analyzed. At median follow-up of 66.8 months, 5-year OS was 72.8% in high-dose group versus 69.9% in low-dose group (p = 0.772) and 5-year DFS 64.7% versus 64.1% (p = 0.871). Patients having early-stage disease receiving low dose and those with advanced disease treated with >30 Gy had better OS and DFS though not statistically significant. Adding rituximab was associated with significantly better OS and DFS irrespective of radiation dose delivered. High IPI score and omitting rituximab were the only factors that significantly worsened both OS and DFS. Acute radiation toxicities were comparable in both groups (p = 0.82). Among late toxicities, no patient developed a second malignancy and 5% died due to cardiovascular complications (p = 0.595) though only two patients (1.1%) had received thoracic radiation.
IntroductionNon-Hodgkin lymphoma (NHL) is the 12th most common cancer in the world as per GLOBOCAN 2020 statistics, accounting for 2.8% of all newly diagnosed cancer cases and 2.6% of cancer related deaths [1]. The standard treatment comprises of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) along with rituximab, anti CD20 antibody. The addition of rituximab has shown improvement in disease-free survival (DFS) and overall survival (OS) in NHL patients [2]. Involved-field radiotherapy (IFRT) is considered for bulky disease and in patients with partial response. Randomized trials by Aviles et al. [3,4] have shown significantly better DFS and OS with combined modality treatment in patients having bulky disease with complete response post chemotherapy and in those with residual disease (<5 cm). Even in the rituximab era, same results were also seen in a retrospective analysis from MD Anderson Cancer Center where addition of radiotherapy (RT) significantly improved OS and DFS in both limited and advanced stage disease with no local relapse within the irradiated field [5]. However, there is no uniformity in the radiation dose, with doses ranging from 30–55 Gy.
NHLs are considered to be radiosensitive tumors requiring much lesser radiation dose as compared to epithelial malignancies. In aggressive NHL, no dose response was seen across 20-50 Gy in an analysis from Stanford University [6]. However, in early-stage NHL, dose dependent response was observed in an analysis from the British National Lymphoma Investigation, with compete response noted at ≥45 Gy [7]. Similarly, the European Organisation for. Research and Treatment of Cancer data showed that risk of local relapse is almost doubled in patients receiving <45 Gy radiation [8]. But, most of these patients did not receive chemotherapy. Hence, in this era of combined modality treatment, the RT dose needs to be carefully evaluated specially in patients who received rituximab. Our aim should be to deliver the lowest compatible dose to achieve optimal efficacy.
The 5-year survival in aggressive NHL was reported to be 58% [9]. Due to their long survival, apart from disease control, treatment related toxicities also are a serious matter of concern. Both acute and late radiation toxicities are dose dependent. Acute reactions depend on the total dose delivered whereas late reactions depend on the total dose as well as the dose per fraction. A retrospective analysis on high-grade NHL patients revealed significantly higher rates of stroke and myocardial infarction in patients receiving ≥40 Gy [10]. Also, delivering a low RT dose comes with logistic benefits, like fewer patient visits, more treatment slots, etc. However, most of the data available is retrospective in nature. There is only one prospective trial by Lowry et al. [11] which highlights dose reduction in both indolent and aggressive NHL does not worsen disease control. In this retrospective analysis, we wanted to see if radiation dose de-escalation is feasible in aggressive NHL treated in the rituximab era.
Materials and MethodsFrom 2007 to 2017, available treatment records of all NHL patients were scrutinised. Of them, only patients with high-grade NHL or diffuse large B-cell lymphoma and those with non-central nervous system primaries were included in the analysis. Written consent was obtained from all the patients. A complete history including comorbidities was noted along with clinical examination. Baseline investigations such as complete blood count, renal and liver function test, lactate dehydrogenase, and histopathology were recorded. All patients underwent computed tomography (CT) of neck, chest, abdomen, and pelvis along with bone marrow biopsy for staging or positron emission tomography (PET)-CT whenever feasible. All of them underwent IFRT with or without chemotherapy. The International Prognostic Index (IPI) was calculated according to the description by the International Non-Hodgkin’s Prognostic Factors Project for patients with all required parameters present and staging as per Ann Arbor classification.
We compared outcome of patients who received ≤30 Gy RT dose (low dose) to those with >30 Gy (high dose). Outcomes compared were response rate, DFS, and OS. The response to treatment was defined as per international workshop criteria [12]. Complete response (CR) was defined as “disappearance of all detectable clinical and radiographic evidence of disease.” Partial response (PR) was defined as “a ≥50% reduction in all measurable tumors.” Progressive disease (PD) was defined as “≥50% increase in the size of previously involved sites or appearance of new lesions despite treatment.” Stable disease (SD) was defined as a response lesser than PR, but not fulfilling the PD criteria.
Toxicities were assessed using the Radiation Therapy Oncology Group grading scale. Acute toxicities were those which occurred within 3 months and late toxicities were those which occurred after 3 months of treatment completion. Acute toxicity was assessed at 1 month of treatment completion. Late toxicities were reported as cumulative events till last follow-up.
Baseline patient and disease characteristics were compared between two groups using chi-square test. OS was calculated from the date of diagnosis till death (due to any cause) or last follow-up and DFS till recurrence, death or last follow-up. DFS and OS were estimated using Kaplan-Meier method with log-rank test. Univariate and multivariate analyses were done to determine the factors affecting disease outcomes, i.e., age, sex stage, IPI, addition of rituximab, and radiation doses delivered. A p-value of <0.05 was considered statistically significant.
ResultsA total of 298 patients with stage I to IV NHL treated with combined modality or radiation alone were analysed. Of them, 98 patients had low-grade NHL, 16 were lost to follow-up. The records of 184 patients were analysed (Table 1). There was no significant difference in the baseline characteristics between the high and low dose groups. The median age of the study population was 50 years (range, 15 to 90 years). The median dose received by patients in low-dose group was 30 Gy (range, 24 to 30 Gy) and high-dose group was 36 Gy (range, 36 to 40 Gy). There was no significant difference in the doses received by patients, either in early or advanced stage. The commonly used dose regimens were 24 Gy in 12 fractions, 30 Gy in 15 fractions, 36 Gy in 18 fractions, and 40 Gy in 20 fractions, irrespective of the disease stage. No significant correlation was observed between disease stage and the prescribed dose (p = 0.63). More than half of them (51.6%) had primary disease in head and neck subsite. Of them, 173 patients (94%) received RT as, or as part of, first-line therapy, with the remaining 6% for relapsed disease. A total of 131 patients (71.2%) had early-stage disease. Of patients having advanced disease receiving RT, 36 (67.9%) had bulky disease, 37 (69.8%) had extra lymphatic involvement, while 28 (52.8%) had PR, 7 (13.2%) SD, and 4 (7.5%) PD.
The median follow-up was 66.8 months (range, 2.8 to 228 months). The response rates after first line treatment-RT ± chemotherapy (initial response) as well as the overall response rates (response at last follow-up) are described in Table 2. There was no statistically significant difference in response rates between the two groups. At the time of analysis, 125 patients (67.9%) were alive. A total of 44 (24%) of the patients expired due to the primary disease itself, 10 (5.4%) owing to cardiovascular disease and rest 5 (2.7%) deaths were related to toxicity of chemotherapy. Of the 10 cardiovascular deaths, seven patients received adriamycin as a part of their combination chemotherapy regimen (5 CHOP and 2 R-CHOP).
The 5-year OS was 72.8% in high-dose group versus 69.9% in low-dose group and 7-year OS was 66.7% versus 65.5%, respectively (p = 0.772). The median OS of the entire study population was not reached. The overall median DFS was 158.99 months (Fig. 1). The DFS rates at 5-year were 64.7% in high-dose group versus 64.1% in low-dose group and at 7-year, it was 62.2% versus 64.1% (p = 0.871). Younger patients had better OS compared to those above 50 years irrespective of the dose delivered. The 5-year OS in the young patients was 75.8% (high dose) versus 74.2% (low dose), while in the elderly group it was 69.2% versus 66.6%. Similar, results were also noted when DFS was compared between these two groups (5-year DFS: 66.1% vs. 70.8% in age ≤50 years, 63% vs. 58.9% in elderly; p = 0.836). Female patients receiving low dose showed better OS and DFS though not statistically significant. Patients having early-stage disease receiving low dose and those with advanced disease treated with high-dose radiation had better OS and DFS though it did not reach statistical significance (5-year OS: 65.6% vs. 42.9% in advanced stage, 75.6% vs. 81.5% in early-stage, p = 0.747; 5-year DFS: 53.1% vs. 38.1% in advanced stage, 69.3% vs. 75.3% in early-stage, p = 0.912) (Fig. 2).
Addition of rituximab was associated with significantly better OS and DFS. The 7-year OS and DFS of those receiving rituximab were 80.6% and 77.8% as compared to those without rituximab, 57.6% and 52.3% (overall, p = 0.003 and p = 0.001, respectively). This benefit of adding rituximab was evident irrespective of the radiation dose delivered (Fig. 3). Better OS and DFS were also noted with lower IPI score although no correlation was observed with radiation doses received.
In low-risk patients (IPI 0 and 1), the 5-year OS was comparable (90% vs. 90.3%), but 5-year DFS was slightly lower in the high-dose group (83.3% vs. 90.3%) (Fig. 4). In patients with IPI scores of 2 and 3, survival was slightly better in low dose group (Fig. 5), 5-year OS 59.6% versus 64.5% (p = 0.931), 5-year DFS 48.1% versus 51.6% (p = 0.57). The median OS of patients having IPI score 3, 4, and 5 was 44.6 months, 10.2 months, and 8.4 months, respectively (p = 0.001). Patients having high IPI scores (4 and 5), high RT dose had better OS and DFS (5-year OS: 14.3% vs. 0%; 5-year DFS: 14.3% vs. 0%) (Fig. 6). Patients with CR had significant better survival than those with non-CR irrespective of the dose group (Fig. 7).
Using Cox regression proportional hazards model to identify the prognostic factors affecting OS and DFS, it was observed that early-stage disease, IPI score, and addition of rituximab were the only significant factors, on univariate analysis but stage did not assume statistical significance in multivariate analysis (Table 3). No significant effect on survival or disease control was observed with reduced RT doses.
Acute radiation induced toxicities showed no statistical significance irrespective of the dose received (p = 0.82). Since about half of the patients received RT to the head and neck region, the most common acute toxicities in both groups, was oropharyngeal mucositis, 19 (16.7%) in high dose versus 9 (12.9%) in low dose, followed by dysphagia 16 (14%) versus 10 (14.3%) and dermatitis 9 (8%) versus 5 (7.1%), respectively. However, the toxicities noted were grade 1–2 only. Only two patients (1.9%) in high-dose group and one (1.6%) in low-dose group experienced grade 3 toxicity (hematological) that required treatment interruption. Other noted acute toxicities were abdominal pain (5 [4.4%] in high dose vs. 4 [5.7%]), diarrhea (6 [5.3%] vs. 4 [5.7%] in low dose), fatigue (10 [8.8%] vs. 6 [8.6%]), respectively. When late toxicities were compared, one (0.8%) patient developed second malignancy. Five patients in each arm died due to cardiovascular complications (4.4% vs 7.1%, p = 0.595). Of them, two (1.7%) in high-dose arm and none in low-dose arm have received RT to mediastinum or cardiac structures. Xerostomia (23 [20.2%] vs. 13 [18.6%]), dry eyes (20 [17.5%] vs. 14 [20%]), and cataract (19 [16.7%] vs. 10 [14.3%]) were the most common late toxicities observed.
Discussion and ConclusionIn this retrospective analysis of 184 patients with high-grade NHL, we observed that delivering RT dose ≤30 Gy does not affect survival as well as local disease control. Disease stage, IPI scores, and inclusion of rituximab in chemotherapy regimen remain the only significant factors which affected both DFS and OS. However, it may be noted that in early-stage disease, low-dose group and in advanced disease, high-dose RT group fared better both in terms of OS and DFS although not statistically significant.
NHL, being a radiosensitive tumor, has a long survival, 10-year OS of 50% [13]. This has led to more emphasis on achieving optimal treatment schedules with minimum acceptable toxicities. With the advent of modern imaging techniques as well as highly conformal RT techniques, the shift from IFRT to involved site radiotherapy and involved node radiotherapy has reduced the irradiated volumes which have led to decreased risk of late toxicities including development of secondary malignancies [14]. Apart from reducing treatment volumes, RT dose de-escalation might also have a potential long-term benefit. In high-grade NHL, the International Lymphoma Radiation Group (ILROG) recommends a dose of 30–36 Gy in PET CR while those with PR, 36–50 Gy is recommended for both primary nodal and extra nodal subtypes [15,16]. Similar doses are also recommended by the National Comprehensive Cancer Network 2022 guidelines for consolidative RT after chemotherapy. If primary RT is given without chemoimmunotherapy, 40–55 Gy should be prescribed [17]. In a phase II trial by Kelsey et al. [18], consolidative RT to a dose of 19.5–20 Gy was delivered. At a median follow-up of 51 months, 5-year OS and progression-free survival (PFS) were 83% and 90%, respectively. However, majority (79%) was early-stage disease and only 28% had bulky disease, both of which are important prognostic factors in NHL. The OS and PFS reported is better than observed in our study population which may be due to addition of rituximab with chemotherapy and utilization of PET-CT for precise response assessment. In another prospective trial by Lowry et al. [11], the overall response rate was 90% with no significant difference in OS, PFS or progression within irradiated field with 30 Gy dose. These results are similar to our observation. The study by Lowry et al. [11] had a major drawback, patients received heterogenous chemotherapy regimens, hence it will be difficult to comment upon the efficacy of consolidative RT in such a situation. In addition, the primary end point of this study was local control instead of OS and PFS, which do not represent treatment efficacy. Based on these studies and our experience, in patients with NHL who achieve clinical or radiological CR after chemotherapy, 30 Gy may be an adequate dose.
Long survival of NHL patients makes treatment induced toxicities, both acute and late, a matter of concern. Second malignancy and cardiopulmonary toxicities have been studied extensively which may be contributed by both RT and chemotherapy (adriamycin, alkylating agents, etc.). In a retrospective analysis on the GELA cohort, the 7-year cumulative incidence of second malignancy was 2.75% [19]. Age was the only significant risk factor and chemoradiotherapy had no significant impact. However, only 4% patients received radiation as a part of their first line management while 18% received RT alone or combined with chemotherapy in refractory or relapse settings. In our study, one (0.8%) patient developed second malignancy (sarcoma) in the RT field, although, he had received combined treatment. Around 2% developed late cardiovascular complications and 0.4% pulmonary toxicities, not influenced by RT. The Surveillance, Epidemiology, and End Results analysis of NHL patients showed significantly higher chances of developing second malignancy compared to endemic rate, although the incidence rates were similar between irradiated and unirradiated patients. The only difference noted was the predominance of solid tumors (sarcoma, breast, and lung cancer) in patients treated with RT [20]. Since RT induced secondary cancers occur in the irradiated field, attempts may be made to lower dose exposure by using modern RT techniques, e.g., deep inspiration breath hold, protons, and use of involved node RT.
Most of our knowledge regarding cardiotoxicity in long-term lymphoma survivors comes from RT delivered 20–30 years ago using mantle field and prescribed doses of ≥40 Gy. Today, radiation portals are significantly smaller and prescribed doses are lower with the advent of chemotherapy. Currently, doses of 20–36 Gy are typically prescribed to a more precisely defined target volume depending on the stage of the disease, type of lymphoma and response to chemotherapy [21]. In this study, we observed 5.4% deaths due to cardiovascular causes, of which only 1.1% patients have received RT to mediastinal or cardiac structures. Compared to the general population, NHL survivors are estimated to be 5.3–7.3 times more prone to develop long-term cardiovascular mortality [22]. However, reduction in treatment volume from mantle field to involved field or node RT has reduced Dmean of heart by 35%–72% [23,24]. A systematic review of cardiotoxicities in patients receiving mediastinal RT showed a linear dose-response relationship between Dmean of heart and death due to cardiac disease, especially when Dmean exceeds 5 Gy [25,26]. Dmean of ≥15 Gy significantly increased chances of symptomatic cardiac injury [27]. The 30-year risk of clinically significant valvular heart disease was estimated to be increased by around 1.4% in patients receiving modern mediastinal RT to a dose of 20–30 Gy [28]. Use of deep inspiratory breath hold along with either three-dimensional conformal radiotherapy, intensity-modulated radiotherapy or even proton therapy further reduces the heart dose [29-31]. With proton therapy, heart-Dmean, V5–30 and heart wall-Dmean could be reduced by ≥30%, left anterior descending artery V5–30 by 11%–28%, Dmean by 72% and mean dose to heart chambers by 47%–100% [32-34]. However, the ILROG recommends use of proton therapy to reduce cardiotoxicity in only two patient subsets; first, patients with mediastinal disease below the origin of the left main coronary artery and secondly in heavily pretreated patients, who are at a higher risk of radiation-related toxicity [35].
A retrospective German study reported acute and late toxicities (xerostomia, dry eyes, cataract, dysphagia, etc.) which were comparable to our report [36]. Of the 75 patients analyzed, 61% received 24–36 Gy radiation while 37% received higher doses. No significant difference in toxicities were observed here with respect to the dose delivered (p = 0.197).
This is a single institute retrospective analysis which included patients from the pre-rituximab era also, 41% received rituximab along with chemotherapy. Most of the patients could not afford rituximab because of financial constraints. Addition of rituximab have been shown to improve the long-term outcomes. There was no effect on the response and outcome with RT in this study.
This retrospective series suggest that RT dose reduction may be possible in high-grade NHL without compromising the tumor control, both short term and long term. Radiation dose de-escalation may be the new standard of care in future for patients receiving RT for NHL in the era of rituximab and PET scan. However, our observations need to be confirmed in a large randomised multicentric trial.
NotesStatement of Ethics This study protocol was reviewed and approved by Postgraduate Institute of Medical Education and Research (No. RT 12/03/23). Written informed consent was obtained from participants to participate in the study. Fig. 4.(A) Overall survival and (B) disease-free survival of IPI scores 0 and 1 in two groups. IPI, International Prognostic Index. 
Fig. 5.(A) Overall survival and (B) disease-free survival of IPI scores 2 and 3 in two groups. IPI, International Prognostic Index. 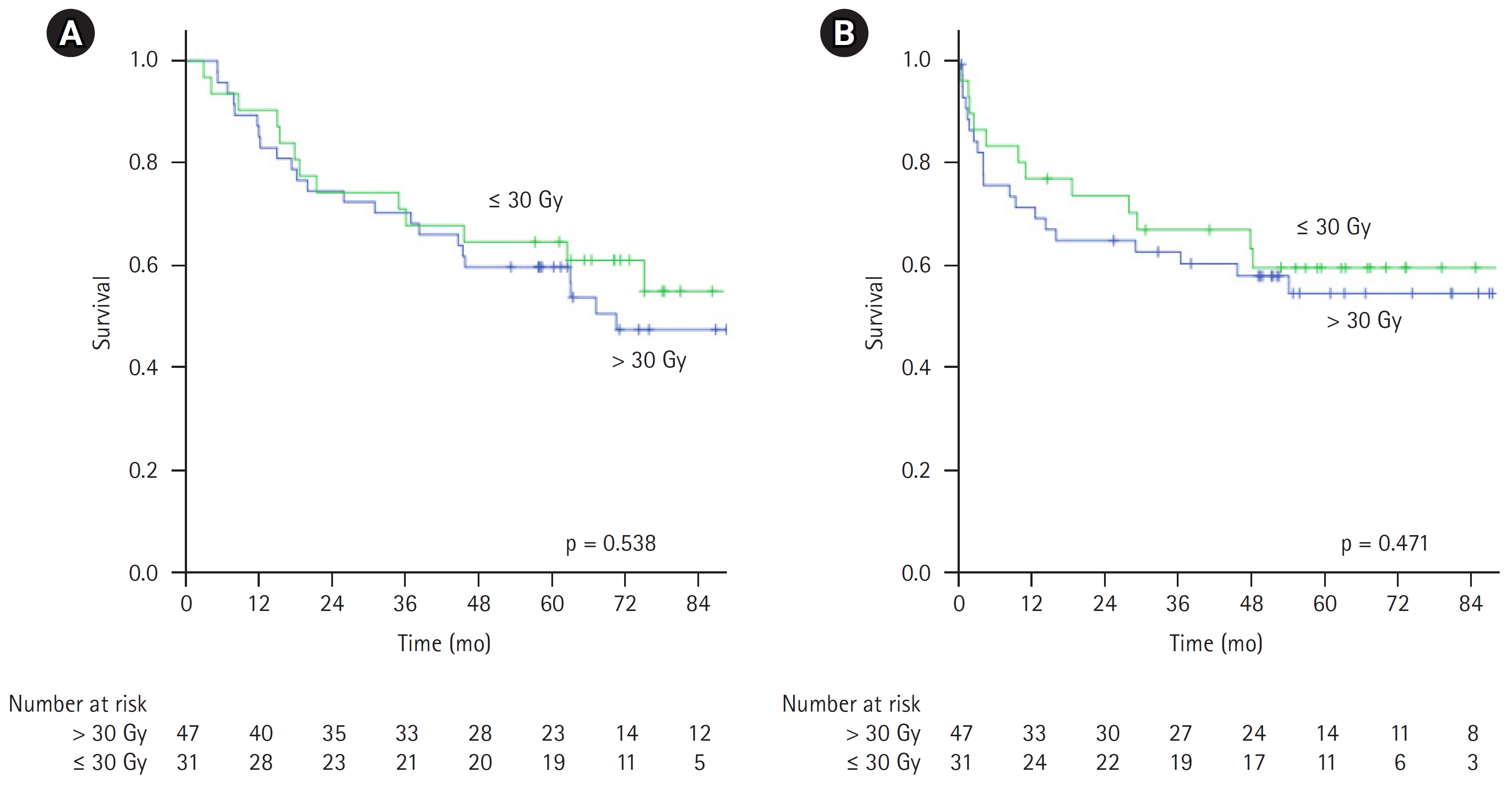
Fig. 6.(A) Overall survival and (B) disease-free survival of IPI scores 4 and 5 in two groups. IPI, International Prognostic Index. 
Table 1.Patient characteristics (n = 184) Values are presented as number (%). RT, radiotherapy; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; R-CHOP, rituximab and CHOP; COP, cyclophosphamide, vincristine, and prednisone. Table 2.Response rates between the two treatment groups Table 3.Prognostic factors affecting OS and DFS References1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49.
2. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomized controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379–91.
3. Aviles A, Fernandezb R, Perez F, et al. Adjuvant radiotherapy in stage IV diffuse large cell lymphoma improves outcome. Leuk Lymphoma 2004;45:1385–9.
4. Aviles A, Neri N, Delgado S, et al. Residual disease after chemotherapy in aggressive malignant lymphoma: the role of radiotherapy. Med Oncol 2005;22:383–7.
5. Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol 2010;28:4170–6.
6. Fuks Z, Kaplan HS. Recurrence rates following radiation therapy of nodular and diffuse malignant lymphomas. Radiology 1973;108:675–84.
7. Lamb DS, Hudson GV, Easterling MJ, MacLennan KA, Jelliffe AM. Localised grade 2 non-Hodgkin’s lymphoma: results of treatment with radiotherapy (BNLI Report No. 24). Clin Radiol 1984;35:253–60.
8. Tubiana M, Carde P, Burgers JM, Cosset JM, Van Glabbeke M, Somers R. Prognostic factors in non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 1986;12:503–14.
9. Issa DE, van de Schans SA, Chamuleau ME, et al. Trends in incidence, treatment and survival of aggressive B-cell lymphoma in the Netherlands 1989-2010. Haematologica 2015;100:525–33.
10. Moser EC, Noordijk EM, van Leeuwen FE, et al. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 2006;107:2912–9.
11. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86–92.
12. Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244.
13. Horvat M, Zadnik V, Juznic Setina T, et al. Diffuse large B-cell lymphoma: 10 years’ real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone. Oncol Lett 2018;15:3602–9.
14. Zwam HM, Habib EE, AL-Daly ME. Involved nodal radiotherapy vs. involved field radiotherapy after chemotherapy in the treatment of early stage Hodgkin’s lymphoma. J Cancer Ther 2013;4:271–9.
15. Wirth A, Mikhaeel NG, Aleman BMP, et al. Involved site radiation therapy in adult lymphomas: an overview of International Lymphoma Radiation Oncology Group guidelines. Int J Radiat Oncol Biol Phys 2020;107:909–33.
16. Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11–31.
17. National Comprehensive Cancer Network. B-cell lymphomas [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022 [cited 2023 Aug 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf.
18. Kelsey CR, Broadwater G, James O, et al. Phase 2 study of dose-reduced consolidation radiation therapy in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys 2019;105:96–101.
19. Andre M, Mounier N, Leleu X, et al. Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood 2004;103:1222–8.
20. Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer 2006;107:108–15.
21. Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2014;89:49–58.
22. Boyne DJ, Mickle AT, Brenner DR, et al. Long-term risk of cardiovascular mortality in lymphoma survivors: a systematic review and meta-analysis. Cancer Med 2018;7:4801–13.
23. Koh ES, Tran TH, Heydarian M, et al. A comparison of mantle versus involved-field radiotherapy for Hodgkin’s lymphoma: reduction in normal tissue dose and second cancer risk. Radiat Oncol 2007;2:13.
24. Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012;83:1232–7.
25. Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010;28:1308–15.
26. Chow EJ, Chen Y, Hudson MM, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol 2018;36:44–52.
27. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606.
28. Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107:djv008.
29. Tomaszewski JM, Crook S, Wan K, Scott L, Foroudi F. A case study evaluating deep inspiration breath-hold and intensity-modulated radiotherapy to minimize long-term toxicity in a young patient with bulky mediastinal Hodgkin lymphoma. J Med Radiat Sci 2017;64:69–75.
30. Aznar MC, Maraldo MV, Schut DA, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys 2015;92:169–74.
31. Paumier A, Ghalibafian M, Gilmore J, et al. Dosimetric benefits of intensity-modulated radiotherapy combined with the deep-inspiration breath-hold technique in patients with mediastinal Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 2012;82:1522–7.
32. Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys 2012;83:260–7.
33. Baues C, Marnitz S, Engert A, et al. Proton versus photon deep inspiration breath hold technique in patients with Hodgkin lymphoma and mediastinal radiation : a planning comparison of deep inspiration breath hold intensity modulation radiotherapy and intensity modulated proton therapy. Radiat Oncol 2018;13:122.
34. Li J, Dabaja B, Reed V, et al. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int J Radiat Oncol Biol Phys 2011;81:167–74.
|
|
||||||||||||||||||||||||||||||||||||||||||

 |
 |