 |
 |
AbstractSurface mould brachytherapy is a conformal radiotherapy technique that can deliver high dose to the target while sparing nearby normal structures, Here, we aim to describe the procedurals details for high-dose rate (HDR) surface mould brachytherapy in sebaceous carcinoma of eyelid in a 54-year old lady. She was hesitant for surgery and any form of invasive intervention like interstitial brachytherapy. So, she was treated with surface mould HDR brachytherapy to a total dose of 52 Gy in 13 fractions at a dose of 4 Gy per fraction delivered twice daily using Iridium-192 isotope with no acute side effects. She was evaluated on a weekly basis for any radiation side effects and now she is disease-free for 6 months post-treatment with only mild dry eye. A detailed step-by-step procedure of surface mould technique, simulation procedure, dose prescription, planning, plan evaluation and treatment has been described in this paper. Surface mould HDR brachytherapy can be safely used as organ preserving modality of treatment for eyelid carcinoma.
IntroductionEyelid carcinoma represents 5%–10% of all skin malignancies with an annual incidence of 15 per 100,000 populations [1,2]. The most common histology is basal cell carcinoma followed by squamous cell carcinoma, sebaceous carcinoma, melanoma, and Merkel cell carcinoma [3,4]. The management of eyelid carcinoma should be discussed in a multidisciplinary tumor board. Surgery is the gold standard of treatment of such malignancy, be it in the form of wide local excision with negative margin or the use of Mohs micrographic surgery [4-6]. But we have to take into consideration the cosmetic outcome and patient’s desire to maintain the functional outcome of the procedure while deciding for the management of carcinoma as a whole. As an alternative to surgery, brachytherapy is a form of radiotherapy that delivers a high dose to the tumor and due to the rapid dose fall-off can spare the normal tissues. The use of brachytherapy for treatment of eyelid carcinoma has resulted in excellent locoregional control with minimal side effects while still maintaining the cosmesis [6-8]. Brachytherapy can be delivered by interstitial technique which is an invasive technique of use of hypodermic needles or in the form of surface mould noninvasive method [9-11]. This study aimed to demonstrate such non-invasive surface mould brachytherapy technique in a step-by-step manner for an easy guide for the clinicians and also to showcase the weekly follow-up post-treatment for any acute reactions.
Case Description1. Case historyA 54-year-old lady with a history of slow-growing mass of size 0.8 cm × 0.5 cm in the right upper eyelid near lateral canthus since 2 years was evaluated by a local ophthalmologist (Fig. 1A). Excisional biopsy from the lesion was taken and the histopathology was reported as a malignant adnexal tumor. There was no comment on margin status. She presented to our outpatient department with the above post-op histopathology report. On local examination, there was a scar in the right upper eyelid with no loss of cilia, no induration, and eyelid margin was normal. There was no palpable cervical lymphadenopathy. Contrast-enhanced magnetic resonance imaging of the face and neck showed no residual disease and no significant lymphadenopathy. On Immunohistochemistry evaluation, the malignant cells were positive for pan-cytokeratin, epithelial membrane antigen, BerEP4, and P63 and negative for carcinoembryonic antigen, androgen receptor, SOX10, S100, and Ki-67 was 30%–40% suggestive of sebaceous carcinoma. Thus the diagnosis was confirmed to be localized sebaceous carcinoma of the upper eyelid.
1) Discussion with patientThe patient and her caregivers were explained about the treatment options such as interstitial or surface mould high-dose rate (HDR) brachytherapy or electron beam therapy and pros and cons of each treatment and the possible side effects to be encountered during and post-treatment. The patient did not want to undertake any invasive procedure and wanted to receive a treatment that had fewer side effects.
2) Tumor board decisionThe case was put in a multi-disciplinary tumor board for discussion regarding the management. As there was no comment on the margin status, it was decided to treat the patient with surface mould HDR brachytherapy. The reason for choosing surface mould brachytherapy over electron beam therapy was that it had a sharp dose fall-off and less dose penetration as compared to electron beam and was non-invasive as compared to interstitial brachytherapy.
3) Counselling of the patientThe patient was counselled regarding the whole procedure of brachytherapy simulation and also the possible side effects to be encountered during and post-treatment and the role of follow-up. After getting consent from the patient the brachytherapy procedure was started as described below.
4) Brachytherapy procedureStep 1 (Mould preparation): The process of brachytherapy starts with the process of mould preparation. Here, two types of eyelid mould were prepared.
One mould was a 5-mm lead that was trimmed according to the shape of the eyelid and is to be placed beneath the eyelid to be treated to protect the underlying organs-at-risk (OARs) from the radiation. This lead was wrapped around with wax to prevent backscatter of radiation to reduce the local reactions and prevent direct contact with ocular structures.
A dummy wax mould with similar size, shape and thickness as that of the lead mould is also prepared. It is to be used only during computed tomography (CT) simulation instead of lead mould to represent lead mould on CT scan and to prevent any artifact due to lead.
Step 2 (CT simulation): During simulation, the patient was set up in the supine position with a neutral neck position. After instilling local anaesthesia into the eye to be treated, the dummy wax mould is placed correctly underneath the eyelid to be treated (Fig. 1B) to cover the eyelid and it was secured with tape. A copper wire is placed and secured with tape over the scar area over the eyelid for proper visualisation of the scar in the CT scan. The patient was then immobilized in this position using a thermoplastic mask. A wax mould of 2 mm is placed over the mask in the region of eye to be treated. Standard flexible catheters were embedded over the wax mould depending on the target volume to be treated and were numbered serially (Fig. 1C). Then, a CT scan was taken from the vertex to clavicle with the catheters in situ and dummy wire inserted into the catheters for easy visualisation in the CT scan. The CT slice thickness of 1 mm was taken. After simulation, the Digital Imaging and Communications in Medicine CT images were sent to our treatment planning system which were then imported for delineation of target and OAR.
Step 3 (Target and OAR delineation): The copper wire (that represented the post-op scar) seen on the CT scan along with a safety margin of 1 cm was taken as the clinical target volume (CTV-scar depicted in Fig. 2A) [12,13]. In brachytherapy, typically no additional margin to the CTV is required to account for the planning target volume as is done in the case of external beam irradiation. Hence, the CTV is required to receive the planned dose. Anterior chamber of the eye, lacrimal gland, lens, eyeball, and the dummy wax (representing the lead mould) are contoured as the OARs (Fig. 2B–2F).
Step 4 (Dose prescription): In low-dose rate (LDR) brachytherapy, a dose of 60 Gy is considered adequate for treatment. The current patient was planned to receive a total dose of 52 Gy in 13 fractions HDR brachytherapy at a dose of 4 Gy per fraction delivered twice daily 6 hours apart to account for repair of sublethal damage. The 2 Gy equivalent dose was 60.67 Gy for the selected dose of 52 Gy HDR and using the Linear-Quadratic model this dose is the same as the 60 Gy of LDR brachytherapy. Therefore, such dose prescription was used for planning.
Step 5 (Radiation planning): In the Oncentra Brachy version 4.6.0 treatment planning system, the catheter reconstruction and numbering and inverse planning simulated annealing algorithm-based planning were done. The 3-dimensional view of the catheters after reconstruction is shown in Fig. 3A for the target and OARs and the source positions.
Step 6 (Plan evaluation):
- Coverage index: The CTV coverage is considered to be adequate when D90 is at least 90% of the prescribed dose [12]. In the current case, the CTV volume is 0.71 mL and the D90 is 4 Gy which is more than 90% of prescription dose. Also, we need to calculate the dose non-uniformity ratio (DNR) which is V100/V150 which should be <0.36 [12]. Here, in this case, the DNR = 24.27/92.2 = 0.26 which is within limits.
- OAR dose statistics: The isodose distribution is shown in Fig. 3B, the dose volume histogram in Fig. 3C. The doses to the OARs (anterior chamber, eyeball, lacrimal gland, and lens) were within tolerable limits as depicted in Fig. 3D.
Step 7 (Treatment delivery): After the plan is approved by both the physician and the physicist, the treatment was delivered in a fractionated manner using Iridium-192 isotope in the Flexitron afterloader machine as planned. Prior to each fraction of treatment, the customized wax-covered lead mould was placed underneath the eyelid to be treated after local anesthesia eye drops, and its proper positioning was confirmed.
Step 8 (Post-treatment care): Post completion of treatment, the patient and her caregiver were clearly explained the role of eye hygiene and were counselled again regarding the probable side effects and the need to follow-up weekly for review and local examination.
DiscussionThe most common management of eyelid carcinoma is surgery but it is frequently associated with functional and cosmetic impairment that may not be acceptable to the patient [6]. As an alternative, surface mould brachytherapy being a non-invasive procedure is more easily acceptable among patients as compared to invasive interstitial technique. Furthermore, it is associated with excellent local control of disease with minimal side effects due to the rapid dose fall-off. The Groupe Européen de Curiethérapie-European SocieTy for Radiotherapy & Oncology working group recommends the use of brachytherapy over external beam therapy as a mode of treatment of skin malignancy having a smaller target volume as a result of excellent dosimetry and good planning perspective with brachytherapy [13]. There is a paucity of literature on brachytherapy treatment of eyelid malignancy as there are no randomized control trials or cohort studies on the same. Only one systematic review was reported to date and that too lacked any randomized controlled trials or cohort studies. This article stated that brachytherapy in eyelid carcinoma was well tolerated among patients with good local control and cosmetic outcomes [14]. An overview of relevant publications on carcinoma of eyelid treated by surface mould brachytherapy is depicted in Table 1 which has been compared with our current study [2,15-17]. This is the first article to report weekly follow-up of the patient for 9 consecutive weeks and the patient reported well with minimal side effects. So, surface mould HDR brachytherapy can be safely used for management of eyelid skin cancers without any panicky situation.
NotesStatement of Ethics All authors declare that written informed consent was obtained from the patient for publication of this case report and accompanying image. Fig. 1.(A) The initial mass of size 0.8 cm × 0.5 cm in the lateral aspect of upper eyelid. (B) Placement of the wax coated mould underneath the eyelid to be treated. (C) Final immobilization mask with the catheters embedded in situ that are serially numbered. 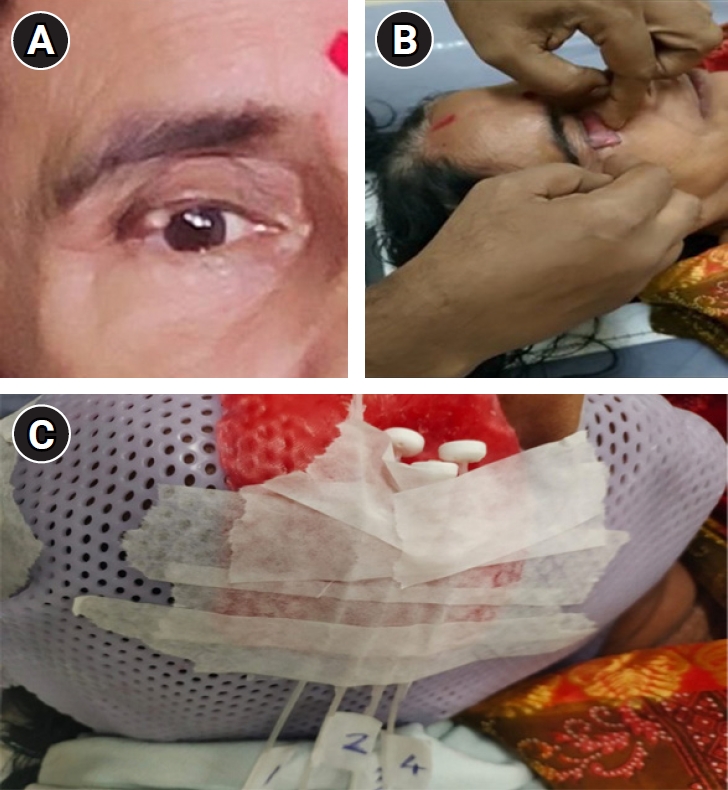
Fig. 2.Computed tomography (CT) scans after CT simulation depicting delineation of target and various organs-at-risk. (A) The delineation of clinical target volume (light blue), (B) anterior chamber (red), (C) lacrimal gland (pink), (D) lens (yellow), (E) eyeball (orange), and (F) wax-covered lead mould (deep blue). 
Fig. 3.(A) The 3-dimensional view of catheters with respect to target and organs-at-risk (OARs) along with source positions marked in red. (B) The isodose distribution in coronal view. (C) The dose volume histogram of the target and OARs. (D) The dose statistics table for the target and OARs. 
Table 1.Comparison of the studies on surface mould brachytherapy for eyelid carcinomas
References1. Yin VT, Merritt HA, Sniegowski M, Esmaeli B. Eyelid and ocular surface carcinoma: diagnosis and management. Clin Dermatol 2015;33:159–69.
2. Vavassori A, Riva G, Durante S, et al. Mould-based surface high-dose-rate brachytherapy for eyelid carcinoma. J Contemp Brachytherapy 2019;11:443–8.
3. Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors: a retrospective study of 5504 cases and review of literature. Am J Dermatopathol 2009;31:256–62.
4. Silverman N, Shinder R. What's new in eyelid tumors. Asia Pac J Ophthalmol (Phila) 2017;6:143–52.
5. Cook BE, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology 2001;108:2088–98.
6. Hata M, Koike I, Maegawa J, et al. Radiation therapy for primary carcinoma of the eyelid: tumor control and visual function. Strahlenther Onkol 2012;188:1102–7.
7. Alam M, Nanda S, Mittal BB, Kim NA, Yoo S. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol 2011;65:377–88.
8. Petsuksiri J, Frank SJ, Garden AS, et al. Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer 2008;112:111–8.
9. Kowalik L, Lyczek J, Sawicki M, Kazalski D. Individual applicator for brachytherapy for various sites of superficial malignant lesions. J Contemp Brachytherapy 2013;5:45–9.
10. Jumeau R, Renard-Oldrini S, Courrech F, et al. High dose rate brachytherapy with customized applicators for malignant facial skin lesions. Cancer Radiother 2016;20:341–6.
11. Azad S, Choudhary V. Treatment results of high dose rate interstitial brachytherapy in carcinoma of eye lid. J Cancer Res Ther 2011;7:157–61.
12. Kovacs G, Martinez-Monge R, Budrukkar A, et al. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update. Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother Oncol 2017;122:248–54.
13. Guinot JL, Rembielak A, Perez-Calatayud J, et al. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol 2018;126:377–85.
14. Frakulli R, Galuppi A, Cammelli S, et al. Brachytherapy in non melanoma skin cancer of eyelid: a systematic review. J Contemp Brachytherapy 2015;7:497–502.
15. Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47:95–102.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |