 |
 |
AbstractPurposeThis scoping review presents the preclinical and clinical data on the effects of high-dose radiation therapy (RT) on bone structure and function.
Materials and MethodAn extensive PubMed search was performed for the relevant questions. The data were then synthesized into a comprehensive summary of the available relevant in-vitro, preclinical and clinical literature.
Results
In-vitro studies of high-dose RT on cell cultures show considerable damage in the viability and functional capacity of the primary cells of the bones; the osteoclasts, the osteoblasts, and the osteocytes. In-vivo animal models show that high-dose RT induces significant morphological changes to the bone, inhibits the ability of bone to repair damage, and increases the fragility of the bone. Clinical data show that there is an increasing risk over time of damage to the bone, such as fractures, after high-dose RT.
IntroductionBones are complex, living tissues. Mature bones maintain their homeostasis, repair damage, and respond to new stresses under the control of both local and systemic processes. All of these processes can be affected by therapeutic doses of ionizing radiation.
Fig. 1 shows the anatomy of a long bone. The following brief description of the anatomy of bone is based upon the detailed presentation in the OpenStax textbook Anatomy and Physiology [1]. The outermost layer of the bone is the periosteum, which is a dense layer of vascular connective tissue. Compact bone is the dense bone beneath the periosteum (also called cortical bone). The Haversian system is a structure within the compact bone wherein the bone is organized into concentric layers, or lamellae, around central canals which contain blood vessels and nerve fibers. These central canals supply nutrients and functional signals to the primary bone cells, the osteocytes. Volkmann's canals are small channels in the bone that transmit blood vessels from the periosteum into the bone and communicate with the Haversian canals. And cancellous bone is the network of trabeculae inside of cortical bone that often contains bone marrow.
Fig. 2 highlights the major cells of bones. There are three primary cells of bones (see the Wikipedia Bone entry [2] or OpenStax textbook [1] for more detail). Osteoblasts are found on the surface of bones. The primary role of osteoblasts is to lay down new bone during growth and remodeling. They secrete collagen and other proteins to form the organic matrix of the bone, the osteoid. Once the osteoid is laid down, osteoblasts initiate the mineralization process. Some osteoblasts are entrapped in the osteoid and mature into osteocytes. Others become flat, inactive cells on the bone surface known as bone lining cells, and others die by apoptosis. Osteoblasts are derived from mesenchymal stem cells. Osteoclasts are large, multinucleated cells originating from the fusion of precursor cells of monocyte/macrophage lineage. The primary function of osteoclasts is bone resorption. They secrete hydrogen ions to dissolve the bone's mineral content and enzymes to break down the organic matrix. Osteocytes are the most abundant cell type in bone, comprising over 90% of all bone cells. They are mature osteoblast cells entrapped in the mineralized matrix of the bone. Osteocytes have a stellate shape with numerous long, thin processes extending out from the through the canaliculi. They participate in the regulation of bone remodeling by controlling the activities of osteoblasts and osteoclasts. They also play a role in phosphorus and calcium homeostasis.
When a bone fractures, the body initiates a repair process [3]. Blood vessels within and around the fracture site rupture, leading to the formation of a hematoma. The hematoma forms a clot that stabilizes the fracture site, provides a temporary framework for healing, and initiates an inflammatory response. The hematoma-triggered inflammatory response induces the influx of immune cells, including neutrophils and macrophages, to the fracture site. Neutrophils remove debris and bacteria, while macrophages phagocytose dead tissue and release growth factors that promote healing. The inflammatory phase typically lasts for a few days. Then, new blood vessels begin to grow into the fracture site, forming granulation tissue. Undifferentiated mesenchymal cells migrate to the fracture site. The mesenchymal cells differentiate into chondroblasts, which produce a soft cartilaginous callus, and osteoblasts, which generate a bone callus. The cartilaginous callus provides temporary stability and helps bridge the fracture gap, while the bone callus provides structural support. The callus formation phase typically lasts for several weeks. As the fracture site becomes more stable, the bone callus undergoes mineralization. Osteoblasts deposit calcium and phosphate minerals, transforming the woven bone into a stronger, more rigid lamellar bone. This initial bone remodeling continues for several weeks to months. Excess bone tissue is removed by osteoclasts and new bone tissue is deposited by osteoblasts. Further remodeling can take months to years, depending on the severity and location of the fracture. Over time, the remodeling returns the bone to its original shape and strength.
Because many patients with cancer receive radiation therapy (RT) that involves the bones, it is important to understand the potential effects of RT on the function and structure of bones. This will aid in minimizing the long-term toxicity of the bones. The objective of this scoping review is to provide a concise synopsis of the effects of RT on bones and the potential effects of RT. This review surveys the relevant pre-clinical and clinical knowledge available on the effects of high-dose radiation therapy on bones. There is a vast literature on both the pre-clinical and the clinical effects, and this review is a presentation of exemplary and significant studies. This review summarizes these articles and synthesizes their potential ramifications for modern RT treatments.
Methods and MaterialsThe recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for the design and presentation of scoping reviews were followed to prepare this report [4]. Multiple PubMed searches were done to identify references for the specific areas being reviewed. The PubMed terms included radiotherapy (as a Medical Subject Headings term) with terms including ŌĆ£AND osteocytes,ŌĆØ ŌĆ£AND osteoblasts,ŌĆØ and ŌĆ£AND osteoclasts.ŌĆØ Other searches included but were not limited to: radiotherapy AND ŌĆ£bone healing,ŌĆØ radiotherapy AND ŌĆ£bone strength,ŌĆØ radiosurgery AND fracture, and radiotherapy AND ŌĆ£sacral fractures.ŌĆØ The identified referencesŌĆÖ abstracts were reviewed, and the relevant articles were obtained. The references of the articles were then reviewed for further relevant references.
Results1. Search resultsUsing the above search methods, 13 papers were selected on the in-vitro effects of high-dose RT on osteoblasts, three papers on the effects on osteoclasts, and two papers on the effects on osteocytes. Another 11 papers were obtained relevant to preclinical animal studies of the effect of RT on bone healing, 13 papers on bone integration, 23 papers on bone morphology and 10 papers on bone strength. For clinical results, systemic reviews or meta-analyses were preferentially selected. Clinical papers obtained on the effects of high-dose radiotherapy on bone included nine papers on dental implants, 16 papers on osteoradionecrosis (ORN), four papers on osteoporosis, 13 papers on sacral insufficiency fractures, 15 papers on compression fractures after stereotactic body radiation therapy (SBRT), and 13 papers on rib fractures after SBRT. These papers were then abstracted and included in the synthesis of this paper.
2. Effect of radiation treatment on in-vitro bone cells1) Osteoclasts
In-vitro studies show that therapeutic radiation doses of x-rays inhibit the function and survival of octeoclasts. For example, Zhang et al. [5] studied the effect of single fraction radiation doses of 1 Gy, 2 Gy, 4 Gy, 6 Gy and 8 Gy on the pre-osteoclast cell line RAW264.7. There was decreased cell viability and decreased activity at doses of Ōēź4 Gy. It is possible that lower doses of RT can stimulate osteoclasts in-vitro. Tong et al. [6] studied the effects of 0.25, 1, and 2 Gy on the same cell line and found that the volume and number of the osteoclasts increased with increasing dose.
2) OsteoblastsMultiple studies show that higher doses of RT decrease osteoblast function and viability [7-11]. For example, Matsumura et al. [7] exposed the osteoblast-like cell line, MC3T3-E1, to doses of 1, 5, and 10 Gy in single fractions. They found that cells irradiated with at least 5 Gy inhibited cell proliferation and induced cell death, as well as inducing osteoblast differentiation to osteocytes. Gal et al. [8] using this MC3T3-E1 cell line, showed that irradiation with 2, 4, or 6 Gy decreased collagen production at doses of 4 and 6 Gy. Szymczyk et al. [9] showed that the decrease in cell proliferation was due to cell death induction by the radiation caused by G2 cell cycle arrest and the resulting enhanced sensitivity to apoptotic factors. Amler et al. [11] isolated human alveolar osteoblasts and expanded them into cell lines. These cell lines were irradiated with single fractions of 2, 6, or 10 Gy. The cells given 2 Gy recovered and functioned normally. The cells given 6 or 10 Gy showed permanent impairment of proliferation and mineralization.
3) OsteocytesOsteocytes are also affected by therapeutic doses of RT. He et al. [12] irradiated the osteocyte-like cell MLO-Y4 with doses of 1, 2, 4, 6, and 8 Gy in single fractions. They noted that the irradiated osteocytes showed morphological changes, including shortened dendrites, inhibited cell viability, and induced apoptosis. These effects increased with the given dose. Wang et al. [13] cultured osteocytes derived from the femurs of BALB/c mice and irradiated the cultures with 2, 4, 6, or 8 Gy single doses. They also showed reduced osteocyte viability with increasing dose, along with changes in dendrite morphology, and induced osteocyte senescence.
3. Pre-clinical animal studies on the effects of radiation on bones1) Bone morphologyRT doses cause damage to the structural integrity of bones. Bakar et al. [14] performed a meta-analysis of eight studies on mice, rats and rabbits between 2015 and 2020 looking at the morphologic changes of bone, such as loss of bone density, after radiation. They concluded that the available studies showed damage from the RT that increased with dose and time from irradiation, and there was a bystander effect on the unirradiated bones. For example, Oest et al. [15] irradiated individual hind legs of mice and monitored the effects on the treated and contralateral untreated femurs. They found a complex reaction to irradiation of 20 Gy in 5 fractions. Over 12 to 26 weeks the irradiated femurs showed trabecular resorption, loss of diaphyseal cortical bone, and decreased bending strength. The contralateral untreated femurs generally followed an intermediate absorption response compared with the treated femurs and placebo-treated femurs, thereby showing a remote bystander effect. Limirio et al. [16] studied the effect of 30 Gy in one fraction to the left hind leg of rats and compared the responses in the treated femurs to the non-irradiated right femur. The measured bone density by CT of both the irradiated femurs (440 Hounsfield unit [HU]) and non-irradiated femurs (480 HU) at 30 days were significantly less dense than the 60-day non-irradiated mouse femur (1,090 HU). The irradiated femur at 60 days was also significantly less dense (880 HU) than the non-irradiated femur.
2) Bone strengthTherapeutic doses of radiation also decrease the strength of bones. Bakar et al.ŌĆÖs meta-analysis [14] also looked at the effect of RT on bone strength. They stated that the mechanical strength was significantly reduced by higher dose irradiation both shortly after and long after the irradiation. The alteration in morphology and strength were related to the quantities and the activities of the osteoblast and osteoclast cells. For example, Perdomo-Oantoja et al. [17] studies the effects of 24 Gy given as a single dose or given as three fractions of 8 Gy on the L5 vertebral body of rabbits. The vertebral bodies given one fraction had lower fracture load strengths and lower stiffness compared to the fractionated bones and unirradiated bones. For all the bones, the bone volume, trabecular number and trabecular spacing correlated with fracture loads and stiffness. In a study by Emerzian [18] the lumbar spines of rats were treated with 30 Gy given as daily 3 Gy fractions for a total of 10 fractions over 2 weeks. The vertebral bodies were harvested and tested 12 weeks after irradiation. She found that the vertebral body strength was 27% lower in the irradiated rats (p < 0.0001). The strength correlated with bone mass and volume.
3) Bone healingRT doses also impede bone healing [19-21]. Among the first reports of the detrimental effect of radiotherapy on bones was Regen et al. [22], who reported in 1936 that 1,100 roentgens of X-rays given prior to a surgical fracture in adult rabbit bones resulted in marked delay of union. Among modern studies on the effects of RT, Arnold et al. [23,24] irradiated rat femurs with single doses of 10 to 22 Gy and then the femurs were surgically fractured 24 hours later. A second set of rats had the radiation 1 week, 10 weeks or 6 months before the surgery, and were treated with single doses of 11 to 20 Gy. Control animals had sham irradiation and then surgery 24 hours later. Formation of new trabecular bone was seen in the sham-irradiated rat femurs at 4 days after surgery and the fracture was almost completely covered with a spongy callus 4 to 5 days later, which then matured to normal bone. At 3 weeks about 50% of the bone was closed, and at 6 weeks 80% was closed. The rat femurs with fractures one day after irradiation showed no adverse effects up to doses of 13 Gy, with possible stimulation of healing at lower doses. Above 13 Gy, the healing was significantly slower in a dose-dependent response. At 7 weeks after 19 Gy less than 30% of the defect was closed. At 30 weeks after 22 Gy less than 20% of the defect was closed. Further, no osteocytes could be detected in the bone. Increasing the interval between the radiation and the fracture to 6 months ameliorated some of the effect, but there was no improvement with this delay of healing for doses >15 Gy. The rat femurs treated with 20 Gy and a 6-month delay of fracture surgery still had almost no healing.
4) Integration effectsAnother example of RT effects is inhibition of a metal implant integration into bone. Jacobsson et al. [25] reported on the dose response of bone integration into a titanium implant in rabbit tibias after single doses of 5, 8, 11, 15, and 25 Gy. At 5 and 8 Gy, bone regeneration was reduced by about 20%. At 11 Gy and above, the bone integration was reduced by about 70%. Dogan et al. [26] implanted four titanium implants into each left tibia of 18 rabbits, and 4 weeks later irradiated the tibias of 12 rabbits with either a single fraction of 15 or 30 Gy. Four weeks after the irradiation the tibias were harvested. The non-irradiated control group showed significantly higher removal torque value than the 15 and 30 Gy irradiation groups. The 15 Gy irradiation group had higher removal torque value than the 30 Gy irradiation group. This was stated to be due to a reduction in the quality of the bone and the bone-to-implant contact area.
4. Clinical effects of RT on bones1) ORN of the mandibleLajolo et al. [27] published a systematic review of ORN after teeth extractions during and after RT, surveying from 1978 to 2021. Among the 462 patients included in the review, 41 developed ORN after extraction. Only three cases were in the maxilla, all in the same 1987 study. All cases of ORN were in papers published in 1987 or earlier. Cespedes-Ajun et al. [28] published a systematic review in 2022 of the incidence of mandibular ORN after intensity-modulated radiation therapy (IMRT) versus three-dimensional conformal radiotherapy (3D-CRT), assuming the IMRT patients would have less dose to the mandible. There was a uniform reduction in the incidence of ORN among the patients treated with IMRT, with ORN rates using 3D-CRT of 5% to 19%, (one paper reported an 87% rate) compared to 0% to 13% with IMRT. In a review by Topkan et al. [29], there was no correlation of radiation dose with ORN of the mandible. A model by van Dijk et al. [30] found that among patient not getting a tooth extraction, keeping the volume of the mandible receiving more than 42 Gy in 2 Gy fractions to less than 30% keeps the risk of ORN to 5% and keeping the 30% mandible volume below 25 Gy lowers this risk to 1%.
2) Dental implantsKende et al. [31] published a systematic review and meta-analysis of papers from 1947 to 2020 on the survival of dental implants in irradiated jaws. They concluded that there was no detrimental effect of irradiation on the success of implantation. Camolesi et al. [32], focusing on reports with 5 year data, found 93% vversus 98% implant survival in irradiated and non-irradiated patients, respectively. Zarzar et al. [33] published in 2023 an umbrella review of meta-analyses on the effects of RT on the success of dental implants. They concluded that implantation in irradiated bones had an 86% success rate versus a 95% success rate in normal bone. Among those studies which separated mandibular implants from maxillary implants, there was a higher failure rate in the irradiated maxillary implants, but not the mandibular implants. However, they only found one high-quality review.
3) Rib fractures after SBRTMa et al. [34] published a pooled analysis of 57 studies incorporating 5,985 cases for chest wall toxicity after SBRT. They found a correlation for rib fractures with sex (female > male), tumor to chest-wall distance, body mass index, the maximum dose to the ribs, and the volume of the 30 Gy dose in the rib (V30). They reported an overall rib fracture rate of 6.3% with a median time to rib fracture of 9 months. Most studies did not have long follow up. Voruganti et al. [35] reported a systematic review of chest wall pain and rib fractures after lung SBRT. They found estimated incidences of chest wall pain and rib fracture of 9% and 5.3%, respectively. The incidence of both correlated with the V30 dose to the chest wall.
4) Sacral insufficiency fracturesRazavian et al. [36] published a systemic review and meta-analysis of radiation-induced pelvic insufficiency fractures, covering 1980 to 2020. A total of 6,488 patients in 37 studies were included, and they found a crude incidence of fractures of 9.4%. In papers with long-term follow-up, the 5-year actuarial incidence was 15.3%. Correlating factors were pre-treatment osteoporosis, diabetes, and post-menopausal state. The most common site of fracture was the sacrum. Sapienza et al. [37], published another meta-analysis on 3,929 patients in 21 studies, focusing on pelvic insufficiency fractures after radiotherapy for gynecologic cancers. Fourteen percent of the patients developed fractures, the most common sites were the sacroiliac joint (34%), sacrum (34%), pubis (13%), and lumbar vertebra (7%). Salcedo et al. [38] reported a prospective trial of 239 women who received pelvic radiotherapy for gynecological cancer from 2008 to 2015. The 1-, 3- and 5-year pelvic fracture rates were 3.6%, 12.7%, and 15.7%. Fractures were associated with baseline osteoporosis, higher baseline bone-specific alkaline phosphatase and age.
5) Vertebral bodiesFaruki et al. [39] published a systematic review on post-operative spine SBRT. They found that the crude vertebral body compression rate was 5.6%. Abbouchie et al. [40] published a systemic review of vertebral body compression after vertebral body SBRT. They found rates of vertebral body compression of 4% to 39%. Risk factors included lytic disease, degree of pre-existing compression, spinal malalignment, increased dose per fraction, and a Spinal Instability Neoplastic Score of >6. Yaprak et al. [41] reported on radiation-induced vertebral body changes after irradiation for gastric cancer. They found a statistically significant decline in bone mineral density in the both the unirradiated and the irradiated vertebral bodies, with a greater loss in the irradiated bones. Kito et al. [42] studied if re-ossification after palliative RT prevented vertebral body fractures. They reviewed the imaging on 111 vertebral body metastases in 54 patients, and most patients had metastatic lung cancer. Re-ossification was seen in 62% of vertebral bodies, with ossification usually seen by 2 months. Vertebral body height reduction was correlated with lack of re-ossification and compression prior to treatment. Re-ossification was associated with the type of cancer (lung cancer patients had a lower rate) and total dose to the vertebral body (for lung cancer, a dose of 20 Gy or less was associated with re-ossification).
6) Other sitesNguyen et al. [43] reported on pathological fractures after SBRT in 505 non-spine bone metastases in 373 patients. The rates of pathological fracture at 6, 12, and 24 months were 3.8%, 6.1%, and 10.9%. Lytic lesions and ribs were more likely to develop fractures. In contrast, in the phase III trial of SBRT for non-spine metastases reported by Nguyen et al. [44], the rate of pathological fracture was 1.2%. However, the primary endpoint, including pathological fracture, was at 3 months and the median follow-up time was not reported.
Discussion and ConclusionThese preclinical and clinical studies show that RT can be detrimental to the health of bones, and that the risk of damage increases with the dose given to the bone. The RT leads to dysfunction and death of the osteocytes and inhibits bone repair by damaging the osteoclasts and osteoblasts. The detrimental clinical effects have long been known. For example, head and neck irradiation can lead to ORN of the mandible and pelvic irradiation can cause pelvic insufficiency fractures. Modern RT techniques in head and neck irradiation and pelvic irradiation are designed to minimize the risk to bones by keeping the dose to the bones as low as possible. However, as RT doses are increasing, especially with the use of SBRT, the incidental dose to bones is increasing. Further, progressively higher single fraction doses are being used for metastases. The doses being clinically used are shown in preclinical studies to cause functional damage or death of the bone. This can lead to an increased risk of fractures. This is reflected by the clinical data. Vargas et al. [45] compared vertebral body fracture rates for SBRT to standard palliative doses. The 5-year fracture rate of SBRT (biological effective dose [BED3] 144 Gy) and standard dose (BED3 80) treatments were 22% and 6.7%. In the study by Nguyen et al. [43] on the fracture rate among non-vertebral body bones treated with SBRT, the rate of fractures at 6, 12, and 24 months were 3.8%, 6.1%, and 10.9%. This study highlights that the damage may be permanent and that there is an increasing risk of fracture over time from treatment. Therefore, short follow-up is insufficient. This is particularly important because there is increased use of SBRT of bone metastases for patients with expected long survival, such as patients with oligometastatic bone-only metastases from breast and prostate cancer [46]. The primary focus in most studies of oligometastatic bone metastases treatment is on survival or short-term decrease in disease progression. However, the long-term risks to bone health have not been extensively reported. There may be a significant risk, based upon these preclinical and clinical studies, of damage to the bones at the high doses being given for SBRT. Alternative approaches, such as fractionated SBRT, may be a safer treatment. Also, minimizing the volume of uninvolved bone being treated, particularly not treating the whole vertebral body, may decrease the risk of fracture.
Osteopenia and osteoporosis increase the risk to bones from RT and are significant problems in the cancer patient population. For example, among men with prostate cancer and no history of androgen deprivation therapy, studies find rates of osteoporosis of 4% to 38%, with more advanced disease having a higher risk of osteoporosis [47]. Men with a history of prostate cancer and androgen deprivation have higher rates of osteoporosis, ranging in various studies from 9% to 53% [48]. Women on aromatase inhibitors for breast cancer are also at increased risk for osteopenia/osteoporosis, with an attendant increased risk of fractures, primarily in the spine [49]. SBRT in these patients may have an increased risk of causing bone fractures from RT. Both preclinical animal studies and clinical studies show that osteoporosis is also a risk factor for developing sacral insufficiency fractures and vertebral body fractures after radiotherapy. It is unknown whether treating the decreased bone mass with bisphosphonates or RANK-L inhibitors could reduce the risk of fractures.
In conclusion, this paper is a representative selection of the available data on the effects of RT on bones. It is not meant to be a definitive summary. It is designed to give an overview of the biology of therapeutic RT on bone, and the possible implications on patients, particularly potential long-term damage. High dose RT has been definitively shown in pre-clinical models to damage or destroy the functional capacity of the cells in the bone. These effects decrease the ability of the bone to heal, decrease the ability of the bone to integrate implants, and decrease the strength of the bone over time. Clinical data, including data on radiation-induced sacral insufficiency fractures after pelvic radiotherapy, vertebral body fractures after SBRT, rib fractures after SBRT, and mandibular ORN, confirm that effects are clinically relevant. With increasing emphasis on higher and higher doses to treat cancers, the doses to the bone may go beyond the therapeutic ratio of the treatment and start to decrease the positive effect of improved local control.
Fig.┬Ā1.The structure of compact bone (source from OpenStax [1], https://openstax.org/books/anatomy-and-physiology-2e/pages/6-3-bone-structure). 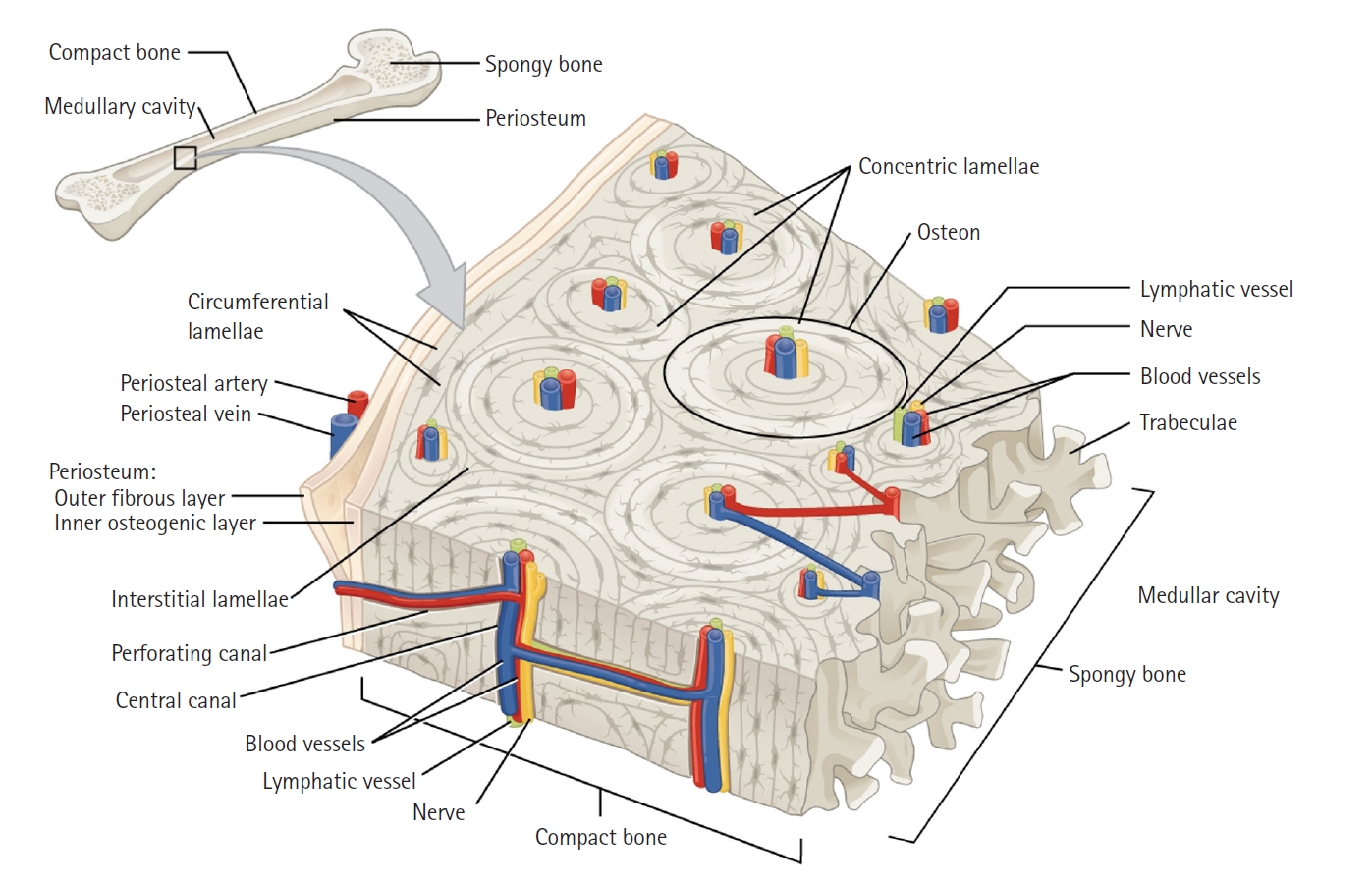
Fig.┬Ā2.The cells of the bones (source from OpenStax [1], https://openstax.org/books/anatomy-and-physiology-2e/pages/6-3-bone-structure). 
References1. OpenStax. Anatomy and physiology 2e: bone structure [Internet]. Houston, TX: OpenStax textbook; 2022 [cited 2024 Jan 30]. Available from: https://openstax.org/books/anatomy-and-physiology-2e/pages/6-3-bone-structure.
2. Wikipedia. Bone [Internet]. San Francisco, CA: Wikipedia Foundation; 2023 [cited 2024 Jan 30]. Available from: https://en.wikipedia.org/w/index.php?title=Bone&oldid=1157832566.
3. Unal M, Akkus O, Marcus RE. Fundamentals of musculoskeletal biomechanics. In : Korkusuz F, editor. Musculoskeletal research and basic science. Cham, Switzerland: Springer; 2016, p. 15ŌĆō36, https://doi.org/10.1007/978-3-319-20777-3_2.
4. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467ŌĆō73.
5. Zhang J, Wang Z, Wu A, et al. Differences in responses to X-ray exposure between osteoclast and osteoblast cells. J Radiat Res 2017;58:791ŌĆō802.
6. Tong L, Zhu G, Wang J, Sun R, He F, Zhai J. Suppressing angiogenesis regulates the irradiation-induced stimulation on osteoclastogenesis in vitro. J Cell Physiol 2018;233:3429ŌĆō38.
7. Matsumura S, Jikko A, Hiranuma H, Deguchi A, Fuchihata H. Effect of X-ray irradiation on proliferation and differentiation of osteoblast. Calcif Tissue Int 1996;59:307ŌĆō8.
8. Gal TJ, Munoz-Antonia T, Muro-Cacho CA, Klotch DW. Radiation effects on osteoblasts in vitro: a potential role in osteoradionecrosis. Arch Otolaryngol Head Neck Surg 2000;126:1124ŌĆō8.
9. Szymczyk KH, Shapiro IM, Adams CS. Ionizing radiation sensitizes bone cells to apoptosis. Bone 2004;34:148ŌĆō56.
10. Park SS, Kim KA, Lee SY, Lim SS, Jeon YM, Lee JC. X-ray radiation at low doses stimulates differentiation and mineralization of mouse calvarial osteoblasts. BMB Rep 2012;45:571ŌĆō6.
11. Amler AK, Schlauch D, Tuzuner S, et al. Pilot investigation on the dose-dependent impact of irradiation on primary human alveolar osteoblasts in vitro. Sci Rep 2021;11:19833.
12. He F, Bai J, Wang J, Zhai J, Tong L, Zhu G. Irradiation-induced osteocyte damage promotes HMGB1-mediated osteoclastogenesis in vitro. J Cell Physiol 2019;234:17314ŌĆō25.
13. Wang Y, Xu L, Wang J, Bai J, Zhai J, Zhu G. Radiation induces primary osteocyte senescence phenotype and affects osteoclastogenesis in vitro. Int J Mol Med 2021;47:76.
14. Bakar AA, Mohamad NS, Mahmud MH, Razak HR, Sudin AE, Shuib S. Systematic review on multilevel analysis of radiation effects on bone microarchitecture. Biomed Res Int 2022;2022:9890633.
15. Oest ME, Policastro CG, Mann KA, Zimmerman ND, Damron TA. Longitudinal effects of single hindlimb radiation therapy on bone strength and morphology at local and contralateral sites. J Bone Miner Res 2018;33:99ŌĆō112.
16. Limirio PH, Soares PB, Emi ET, et al. Ionizing radiation and bone quality: time-dependent effects. Radiat Oncol 2019;14:15.
17. Perdomo-Pantoja A, Holmes C, Lina IA, et al. Effects of single-dose versus hypofractionated focused radiation on vertebral body structure and biomechanical integrity: development of a rabbit radiation-induced vertebral compression fracture model. Int J Radiat Oncol Biol Phys 2021;111:528ŌĆō38.
18. Emerzian SR. Role of radiation treatment on bone strength and fracture risk [dissertation]. Berkeley, CA: University of California; 2021.
19. Pelker RR, Friedlaender GE, Panjabi MM, Kapp D, Doganis A. Radiation-induced alterations of fracture healing biomechanics. J Orthop Res 1984;2:90ŌĆō6.
20. Takahashi S, Sugimoto M, Kotoura Y, et al. The effects of intraoperative radiotherapy on bone-healing ability in relation to different doses and postradiotherapy intervals. Int J Radiat Oncol Biol Phys 1994;30:1147ŌĆō52.
21. Lerouxel E, Moreau A, Bouler JM, et al. Effects of high doses of ionising radiation on bone in rats: a new model for evaluation of bone engineering. Br J Oral Maxillofac Surg 2009;47:602ŌĆō7.
22. Regen EM, Wilkins WE. The influence of roentgen irradiation on the rate of healing of fractures and the phosphatase activity of the callus of adult bone. J Bone Joint Surg 1936;18:69ŌĆō79.
23. Arnold M, Kummermehr J. Radiation induced damage to the regenerative capacity of surgically traumatized rat femur after single doses of X-rays. In : McCormack PD, Swenberg CE, Bucker H, editors. Terrestrial space radiation and its biological effects. Boston, MA: Springer; 1988, p. 475ŌĆō86.
24. Arnold M, Kummermehr J, Trott KR. Radiation-induced impairment of osseous healing: quantitative studies using a standard drilling defect in rat femur. Radiat Res 1995;143:77ŌĆō84.
25. Jacobsson M, Jonsson A, Albrektsson T, Turesson I. Dose-response for bone regeneration after single doses of 60Co irradiation. Int J Radiat Oncol Biol Phys 1985;11:1963ŌĆō9.
26. Dogan GE, Halici Z, Karakus E, Erdemci B, Alsaran A, Cinar I. Dose-dependent effect of radiation on resorbable blast material titanium implants: an experimental study in rabbits. Acta Odontol Scand 2018;76:130ŌĆō4.
27. Lajolo C, Rupe C, Gioco G, et al. Osteoradionecrosis of the jaws due to teeth extractions during and after radiotherapy: a systematic review. Cancers (Basel) 2021;13:5798.
28. Cespedes-Ajun CA, Amghar-Maach S, Gay-Escoda C. Incidence of mandibular osteoradionecrosis (MORN) after intensity modulated radiotherapy (IMRT) versus 3D conformal radiotherapy (3D-CRT): a systematic review. Med Oral Patol Oral Cir Bucal 2022;27:e539ŌĆō49.
29. Topkan E, Kucuk A, Somay E, Yilmaz B, Pehlivan B, Selek U. Review of osteoradionecrosis of the jaw: radiotherapy modality, technique, and dose as risk factors. J Clin Med 2023;12:3025.
30. van Dijk LV, Abusaif AA, Rigert J, et al. Normal tissue complication probability (NTCP) prediction model for osteoradionecrosis of the mandible in patients with head and neck cancer after radiation therapy: large-scale observational cohort. Int J Radiat Oncol Biol Phys 2021;111:549ŌĆō58.
31. Kende PP, Ranganath S, Landge JS, et al. Survival of dental implants on irradiated jaws: a systematic review and meta-analysis. J Maxillofac Oral Surg 2022;21:787ŌĆō95.
32. Camolesi GC, Veronese HR, Celestino MA, et al. Survival of osseointegrated implants in head and neck cancer patients submitted to multimodal treatment: a systematic review and meta-analysis. Support Care Cancer 2023;31:641.
33. Zarzar AM, Sales PH, Barros AW, Arreguy IM, Carvalho AA, Leao JC. Effectiveness of dental implants in patients undergoing radiotherapy for head and neck cancer: an umbrella review. Spec Care Dentist 2023.
34. Ma JT, Liu Y, Sun L, et al. Chest wall toxicity after stereotactic body radiation therapy: a pooled analysis of 57 studies. Int J Radiat Oncol Biol Phys 2019;103:843ŌĆō50.
35. Voruganti IS, Donovan E, Walker-Dilks C, Swaminath A. Chest wall toxicity after stereotactic radiation in early lung cancer: a systematic review. Curr Oncol 2020;27:179ŌĆō89.
36. Razavian N, Laucis A, Sun Y, et al. Radiation-induced insufficiency fractures after pelvic irradiation for gynecologic malignancies: a systematic review. Int J Radiat Oncol Biol Phys 2020;108:620ŌĆō34.
37. Sapienza LG, Salcedo MP, Ning MS, et al. Pelvic insufficiency fractures after external beam radiation therapy for gynecologic cancers: a meta-analysis and meta-regression of 3929 patients. Int J Radiat Oncol Biol Phys 2020;106:475ŌĆō84.
38. Salcedo MP, Sood AK, Jhingran A, et al. Pelvic fractures and changes in bone mineral density after radiotherapy for cervical, endometrial, and vaginal cancer: a prospective study of 239 women. Cancer 2020;126:2607ŌĆō13.
39. Faruqi S, Chen H, Fariselli L, et al. Stereotactic radiosurgery for postoperative spine malignancy: a systematic review and international stereotactic radiosurgery society practice guidelines. Pract Radiat Oncol 2022;12:e65ŌĆō78.
40. Abbouchie H, Chao M, Tacey M, et al. Vertebral fractures following stereotactic body radiotherapy for spine metastases. J Med Imaging Radiat Oncol 2020;64:293ŌĆō302.
41. Yaprak G, Gemici C, Temizkan S, Ozdemir S, Dogan BC, Seseogullari OO. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018;18:972.
42. Kito M, Tsukahara Y, Okamoto M, et al. Does re-ossification after palliative radiotherapy for spinal bone metastases help maintain vertebral body height? Spine J 2023;23:1540ŌĆō8.
43. Nguyen EK, Korol R, Ali S, et al. Predictors of pathologic fracture and local recurrence following stereotactic body radiation therapy to 505 non-spine bone metastases. Radiother Oncol 2023;186:109792.
44. Nguyen QN, Chun SG, Chow E, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: a randomized phase 2 trial. JAMA Oncol 2019;5:872ŌĆō8.
45. Vargas E, Susko MS, Mummaneni PV, Braunstein SE, Chou D. Vertebral body fracture rates after stereotactic body radiation therapy compared with external-beam radiation therapy for metastatic spine tumors. J Neurosurg Spine 2020;33:870ŌĆō6.
46. Rogowski P, Roach M, Schmidt-Hegemann NS, et al. Radiotherapy of oligometastatic prostate cancer: a systematic review. Radiat Oncol 2021;16:50.
47. Lassemillante AC, Doi SA, Hooper JD, Prins JB, Wright OR. Prevalence of osteoporosis in prostate cancer survivors II: a meta-analysis of men not on androgen deprivation therapy. Endocrine 2015;50:344ŌĆō54.
|
|
|||||||||||||||||||||||||||||||||||||||||||||

 |
 |