 |
 |
AbstractPurposeWe investigated the role of radiotherapy (RT) for pancreatobiliary neuroendocrine tumors (PB-NETs).
Materials and MethodsWe identified 9 patients with PB-NETs who received RT between January 2005 and March 2012. Of these 9 patients, 4 were diagnosed with NETs in the pancreas and 5 were diagnosed with NETs in the gallbladder. All patients received RT to the primary tumor or resection bed with a median total irradiation dose of 50.4 Gy, with or without chemotherapy.
ResultsThe tumor response rate and tumor control rate in the RT field were 60% and 100 %, respectively. All 4 patients who underwent surgery had no evidence of disease in the RT field. Of the 5 patients who received RT to the primary gross tumor, 1 had complete response, 2 had partial response, and 2 had stable disease in the RT field. The median time to progression was 11 months. Of the 9 patients, four patients had no progression, and 5 patients had progression of disease (locoregional, 2; distant, 2; locoregional/distant, 1). Of the 4 patients without progression, 3 were treated with RT in adjuvant or neoadjuvant setting, and one received RT to primary tumor. One patient experienced radiation-induced duodenitis at 3 months after concurrent chemoradiation without treatment-related mortality.
IntroductionPancreatobiliary neuroendocrine tumors (PB-NETs) are uncommon malignancies arising from the neuroendocrine system of the pancreatobiliary tract. The annual incidence is estimated at <5 in 1,000,000 in Asian, United States, and European studies [1-5]. Improvement of diagnostic tools and growing awareness of gastrointestinal NETs have resulted in increased detection, and thus, increased incidence of PB-NETs worldwide. Halfdanarson et al. [6] reported an annual incidence of 2.2 in 1,000,000, covering a period of 27 years, with a higher incidence in PB-NETs in recent decades. In Korea, Cho et al. [7] reported that the incidence of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) has shown a remarkable increase during the last decade and the incidence of PB-NETs is 9.5% of all GEP-NETs.
For treatment of PB-NETs, a multidisciplinary approach including surgical resection and chemotherapy is acceptable [8], but advanced PB-NETs still remain a difficult therapeutic challenge because of their high malignant potential and resistance to conventional chemotherapy with etoposide, platinum agents, anthracyclines, streptozocin, and 5-fluorouracil (5-FU) based agents [1,9]. Thus, there is not yet a consensus on appropriate therapeutic approaches in the management of PB-NETs. Radiotherapy (RT), or combined chemoradiation (CRT), has not been widely used in the management of PB-NETs, and also has not been incorporated into multidisciplinary management of PB-NETs.
In this study, we retrospectively analyzed clinical outcomes to investigate the potential role of RT for PB-NETs patients. This study provided a single institution experience of patients with PB-NETs who were treated with external beam radiation (EBRT) intended for curative purposes.
Materials and Methods1. Patient eligibilityWe included patients diagnosed with PB-NETs who were treated with RT with or without chemotherapy at our institution between January 2005 and March 2012. We identified 9 patients (7 males, 2 females) who had a pathological diagnosis of PB-NETs and who received EBRT to the primary tumor or resection bed.
2. Radiotherapy techniqueAll patients received RT to the primary tumor or resection bed for a total irradiated dose of 45 Gy or above, with or without chemotherapy. All patients received 3-dimensional conformal radiotherapy with energies ‚Č•6 MV generated by a linear accelerator or intensity modulated radiation therapy (IMRT) by tomotherapy HI-ART II (TomoTherapy Inc., Madison, WI, USA). The treatment volume consisted of the gross tumor volume, defined by pancreatic and locoregional radiographic abnormalities identified by contrast-enhanced computed tomography (CT), the clinical target volume, defined as the area at risk for subclinical microscopic disease, and the planning target volume, typically consisting of a 0.5-cm margin outside of the clinical target volume. The RT technique consisted of multi-field techniques. If the patients received RT to the primary tumor, after a dose of 41.4 Gy in 23 fractions, the field was reduced to protect the critical organ and then an additional boost of 5.4 to 9.0 Gy was delivered in fractions of 1.8 Gy.
3. Treatment outcome assessmentPatients were considered assessable if measurable disease on CT scan or positron emission tomography-CT was present prior to the initiation of treatment. Response to treatment in the RT field was evaluated using revised Response Evaluation Criteria in Solid Tumors (ver. 1.1) criteria [10], with complete response (CR) defined as the disappearance of the all target lesions, partial response (PR) defined as at least a 30% decrease in the sum of diameters of target lesions and progressive disease (PD) defined as at least a 20% increase in the sum of diameters of target lesions. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Additionally, no evidence of disease (NED) was defined as no objective evidence of tumor recurrence for patients treated with surgery. The tumor response rate for primary tumor was the proportion of CR and PR, and tumor control rate for primary tumor or resection bed was the proportion of NED, CR, PR, and SD. Local failure was defined as tumor recurrence in the primary site, regional failure was tumor recurrence in the regional lymph nodes, and recurrences at other sites were regarded as distant failure. Survival was defined as the time from the date of initiation of RT to the date of death as a result of any cause. Time to progression (TTP) was defined as the time from the date of initiation of RT to the first objective documentation of tumor progression or to the time of death as a result of PD in the absence of previous documentation of objective PD.
4. Toxicity assessmentWe defined acute toxicities as events occurring within 3 months from the start of RT. Late toxicities were defined as events occurring after 3 months from the start of RT. All toxicities were graded according to the Common Terminology Criteria for Adverse Events (ver. 3.0) and toxicities rated higher than grade 3 were considered severe.
Results1. Patient and treatment characteristicsThe median age at the time of initiation of treatment was 54 years (range, 38 to 61 years). Seven patients (77.8%) were men. All patients had the Eastern Cooperative Oncology Group performance of 0 or 1. Of the 9 patients, 4 patients were diagnosed with NET in the pancreas and 5 patients were diagnosed with NET in the gallbladder. Prior to treatment, 7 patients had locally advanced NET, 1 patient had adjacent liver metastasis, and 1 patient had para-aortic lymph node and adjacent liver metastasis. Six patients were diagnosed with poorly differentiated NETs. Among 9 patients, 4 underwent surgical resection. One patient was treated with preoperative RT followed by surgery and 3 patients were treated with surgery followed by postoperative RT. All four patients who underwent surgical resection had tumor resection with negative margins.
The median of the total irradiation dose was 50.4 Gy (range, 45.0 to 58.4 Gy). All patients completed the full course of RT with or without chemotherapy at the intended dose without dose reduction. Six patients received EBRT by a linear accelerator from 45.0 to 50.4 Gy at 1.8 Gy daily, 5 times a week, and 3 patients were treated with IMRT by tomotherapy from 55.7 to 58.4 Gy at 2.2 to 2.9 Gy daily, 5 times a week. Of the 9 patients, 7 received a combination of concurrent CRT, and 2 patients received RT alone. Four patients were treated with etoposide plus carboplatin, 2 patients were treated with 5-FU, and 1 patient was treated with etoposide, and carboplatin plus 5-FU with concurrent RT. Five patients were treated with CRT to primary tumor, and 4 patients underwent resection with adjuvant CRT/RT or neoadjuvant CRT. The patient and treatment characteristics are summarized in Table 1.
2. Treatment outcomeOf the 9 patients, four patients (45%) underwent surgery with NED in the RT field, despite absence of additional treatment. Of the remaining 5 patients, three patients (33%) who received concurrent CRT were diagnosed with locally advanced PB-NETs with or without distant metastasis and experienced CR or PR in the RT field. Fig. 1 illustrates the regression in tumor size of patient #5. Two (22%) of the 5 patients demonstrated SD in the RT field. The tumor response rate for primary tumor and tumor control rate for primary tumor or resection bed were 60% (20% CR, 40% PR, and 40% SD) and 100% (45% NED, 11% CR, 22% PR, and 22% SD), respectively.
The median follow-up period for surviving patients was 16 months (range, 13 to 59 months) and the median TTP was 11 months (range, 2 to 59 months). Four patients were alive without progression, 1 patient was alive with progression, and 4 patients were dead with progression. Progression of disease occurred in 5 patients with 2 locoregional failures, 2 distant failures, and 1 synchronous locoregional and distant failure. Three of 4 patients underwent surgery and one of 5 patients who received concurrent CRT were alive without disease. One patient (patient #6) who survived 5 years from preoperative concurrent CRT followed by surgery without tumor recurrence was diagnosed initially with locally advanced well differentiated NET without lymph node metastasis in the pancreas. The prominent pattern of mortality in patients was not locoregional failure, but distant metastasis. The treatment outcomes with survival status are shown in Table 2.
3. ToxicityThe three patients experienced acute hematologic toxicities above grade 3. Two patients had grade 3 neutropenia and 1 had grade 3 thrombocytopenia during concurrent CRT. No acute non-hematologic grade 3 and 4 toxicities were observed. Late toxicity was observed in one patient who experienced grade 3 radiation-induced duodenitis at 3 months after concurrent CRT. There were no treatment-related deaths.
Discussion and ConclusionRT has not historically played a major role in the treatment of PB-NETs. This study included a series of patients with PB-NETs treated with curative-intent RT for primary gross disease, or after resection. Our findings show that RT could be an optional treatment modality for achieving local control in management of PB-NETs. RT produced excellent tumor control rate and yielded local control in patients with advanced PB-NETs. In terms of toxicity associated with this treatment, 3 patients had acute hematologic grade 3 toxicities and 1 patient had late gastrointestinal grade 3 toxicities. However, there were no treatment-related deaths.
A review of the literature showed that the role of RT in the management for PB-NETs had not been extensively evaluated because of the low incidence of this tumor. Few reports have described RT use for PB-NETs. Strosberg et al. [11] reported a study in which 6 patients were treated with a combination of concurrent and sequential CRT for treatment of locally advanced pancreatic NETs. The radiologic response rate was 80% of patients and all patients tolerated the CRT. Torrisi et al. [12] reported 2 patients with locally advanced pancreatic NETs, 1 of whom was treated with a combination of intraoperative iodine-125 brachytherapy followed by 41.4 Gy EBRT, and the other patient was treated with a combination of EBRT to a dose of 45 Gy and chemotherapy. The first patient achieved a prolonged PR of over 4 years, whereas the second patient achieved a PR lasting 7 months and then had recurrence. Rich [13] described a patient with pancreatic NET who received 46.5 Gy EBRT and had resolved local symptoms for 39 months until the local tumor recurred. The patient died 61 months from the start of irradiation, despite subsequent chemotherapy consisting of 5-FU and streptozocin. Recently, Saif et al. [14] reported that all 6 patients with pancreatic NET treated with concurrent CRT consisting of capecitabine or 5-FU experienced clinical benefit and PR. One patient survived 9 years from treatment and another patient was alive at 4.5 years. These studies documenting clinical outcomes from RT in patients with PB-NETs have suggested that PB-NETs are not radioresistant, but are radioresponsive [11-15].
In our series, both locoregional failure and distant failure in patients receiving CRT/RT in adjuvant or neoadjuvant setting were higher than those who received CRT/RT to primary gross disease. In the adjuvant or neoadjuvant setting, patients of PB-NETs treated with curative-intent RT had 25% locoregional failure and no distant failure. And patients who received concurrent CRT to the primary gross tumor had 40% locoregional failure and 60% distant failure. Zagar et al. [15] from the Duke University reviewed the 33 resected pancreatic NETs patients treated with or without RT in the adjuvant or neoadjuvant setting. Of the 33 patients, sixteen patients were treated with surgical resection alone, while 17 underwent resection with adjuvant or neoadjuvant RT. The 2-year local control rate was 85% for surgery with RT group and 90% for the surgery alone group (p = 0.38). The authors concluded that the role of RT in the adjuvant management of pancreatic NETs remains unclear. However, no statistically significant difference of local control seen between both groups could be explained by factor that the potential probability of surgery with RT group had more patients with an aggressive tumor biology/more locally aggressive disease patients compared with the surgery group. Solorzano et al. [16] reported that, of patients with pancreatic NETs who undergo surgical resection with curative intent, 50% will develop a recurrence within 5 years. In present study, the finding that RT in the adjuvant setting had a 75% locoregional control rate without distant recurrence is useful information in the treatment of this disease. A summary of published studies with PB-NETs patients treated with RT are shown in Table 3.
There have been several studies that used a targeted peptide receptor analog in the management of advanced or metastatic NETs to improve clinical responses. Kwekkeboom et al. [17] reported the GEP-NETs showed high response rates to the somatostatin analog octreotate. The recent success of the biologic targeted agents, such as everolimus, sunitinib and endostatin, has renewed excitement about treating advanced pancreatic NETs [18-20]. Biologic targeted agents have anti-angiogenic properties, whereas RT is known to block the growth of tumor vasculature. So, the combination of targeted agents and EBRT might be an effective treatment for this disease based on the rationale that PB-NETs are highly vascular tumors.
Our study was limited because the analysis was retrospective and the number of patients was small, but there are very few studies on the treatment outcome of RT for PB-NETs because of the low incidence of this tumor and the low therapeutic application of RT for this disease. For all those reasons, prognostic parameter related progression and survival were unable to analysis in this study.
In conclusion, our experience provide that RT with or without chemotherapy can be used as an effective treatment modality in the management of advanced PB-NETs. RT may induce tumor response/control and achieve local control of PB-NETs. The future role of RT in combination with chemotherapy or biologic modifiers for the treatment of PB-NETs remains to be established. We suggest that RT should be considered as a part of multimodality therapy in curative management of advanced PB-NETs.
References1. Buchanan KD, Johnston CF, O'Hare MM, et al. Neuroendocrine tumors: a European view. Am J Med 1986;81:14‚Äď22, PMID: 2879446.
2. Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol 1997;23:36‚Äď42, PMID: 9066745.
3. Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol 2010;21:1794‚Äď1803, PMID: 20139156.
4. Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204‚Äď2210, PMID: 11596039.
5. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1‚Äď18, PMID: 21349409.
6. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727‚Äď1733, PMID: 18515795.
7. Cho MY, Kim JM, Sohn JH, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat 2012;44:157‚Äď165, PMID: 23091441.
8. Kulke MH, Benson AB 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw 2012;10:724‚Äď764, PMID: 22679117.
9. Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519‚Äď523, PMID: 1310159.
10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228‚Äď247, PMID: 19097774.
11. Strosberg J, Hoffe S, Gardner N, Choi J, Kvols L. Effective treatment of locally advanced endocrine tumors of the pancreas with chemoradiotherapy. Neuroendocrinology 2007;85:216‚Äď220, PMID: 17541257.
12. Torrisi JR, Treat J, Zeman R, Dritschilo A. Radiotherapy in the management of pancreatic islet cell tumors. Cancer 1987;60:1226‚Äď1231, PMID: 3040209.
13. Rich TA. Radiation therapy for pancreatic cancer: eleven year experience at the JCRT. Int J Radiat Oncol Biol Phys 1985;11:759‚Äď763, PMID: 2984152.
14. Saif MW, Ng J, Chang B, Russo S. Is there a role of radiotherapy in the management of pancreatic neuroendocrine tumors (PNET)? JOP 2012;13:174‚Äď176, PMID: 22406594.
15. Zagar TM, White RR, Willett CG, et al. Resected pancreatic neuroendocrine tumors: patterns of failure and disease-related outcomes with or without radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1126‚Äď1131, PMID: 22270161.
16. Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery 2001;130:1078‚Äď1085, PMID: 11742342.
17. Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23:2754‚Äď2762, PMID: 15837990.
18. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514‚Äď523, PMID: 21306238.
19. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501‚Äď513, PMID: 21306237.
20. Kulke MH, Bergsland EK, Ryan DP, et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol 2006;24:3555‚Äď3561, PMID: 16877721.
Fig. 1Abdominal computed tomography scans of 51-year-old male patient diagnosed as having gallbladder neuroendocrine tumor with lymph node involvement and adjacent liver metastasis (arrow) treated with radiotherapy (RT) to gross mass: (A) 1 month before RT, (B) 1 month after RT, and (C) 12 months after RT. 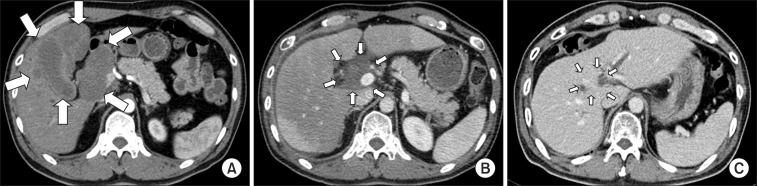
Table 1Patient and treatment characteristics 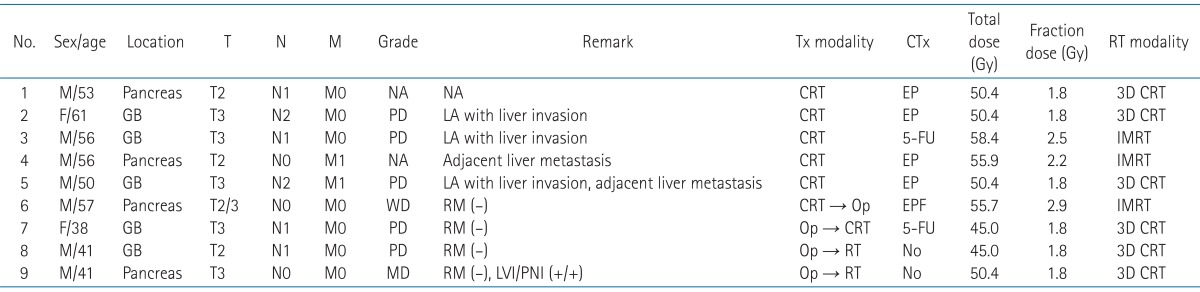 T, tumor; N, node; M, metastasis; Tx, treatment; CTx, chemotherapy; RT, radiotherapy; NA, not applicable; GB, gallbladder; LA, locally advanced; EP, etoposide and carboplatin; RM, resection margin; 5-FU, 5-fluorouracil; CRT, chemoradiation; Op, operation; IMRT, intensity modulated radiation therapy; LVI, lymphovascular invasion; PNI, perineural invasion. Table 2Treatment outcomes of radiotherapy for pancreatobiliary neuroendocrine tumors  TTP, time to progression; OS, overall survival; SD, stable disease; DM, distant metastasis; LN, lymph node; CR, complete response; PR, partial remission; PD, progressive disease; NED, no evidence of disease; L, local recurrence; R, regional recurrence; DWD, died with disease; AWD, alive with disease. |
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |