Introduction
The abscopal effect (from the Latin ab- for “away from” and scopus for “target”) is the regression of tumors at a distance from the irradiated lesions; it was first described in 1953 [1]. In the last several decades, the abscopal effect has been discussed by radiation oncologists as an interesting but extremely rare phenomenon with little hope for application in real practice [2]. The abscopal effect is mediated by immunologic mechanisms. In the immunotherapy era, a new appreciation of the role played by anti-cancer immune systems in orchestrating the anti-tumor effects of radiotherapy (RT) has caused a major spike in interest in the abscopal effect [3]. Here, we have reported a case of metastatic luminal B breast cancer showing the abscopal effect after multi-site five-fraction palliative RT and proposed some ways to increase the probability of occurrence of the abscopal effect.
Case Report
After approval by the Institutional Review Committee of Severance Hospital (No. 4-2020-0947), the medical records of patients with breast cancer treated at our institution were examined. A patient who received intensity-modulated radiotherapy (IMRT) was identified and selected for study.
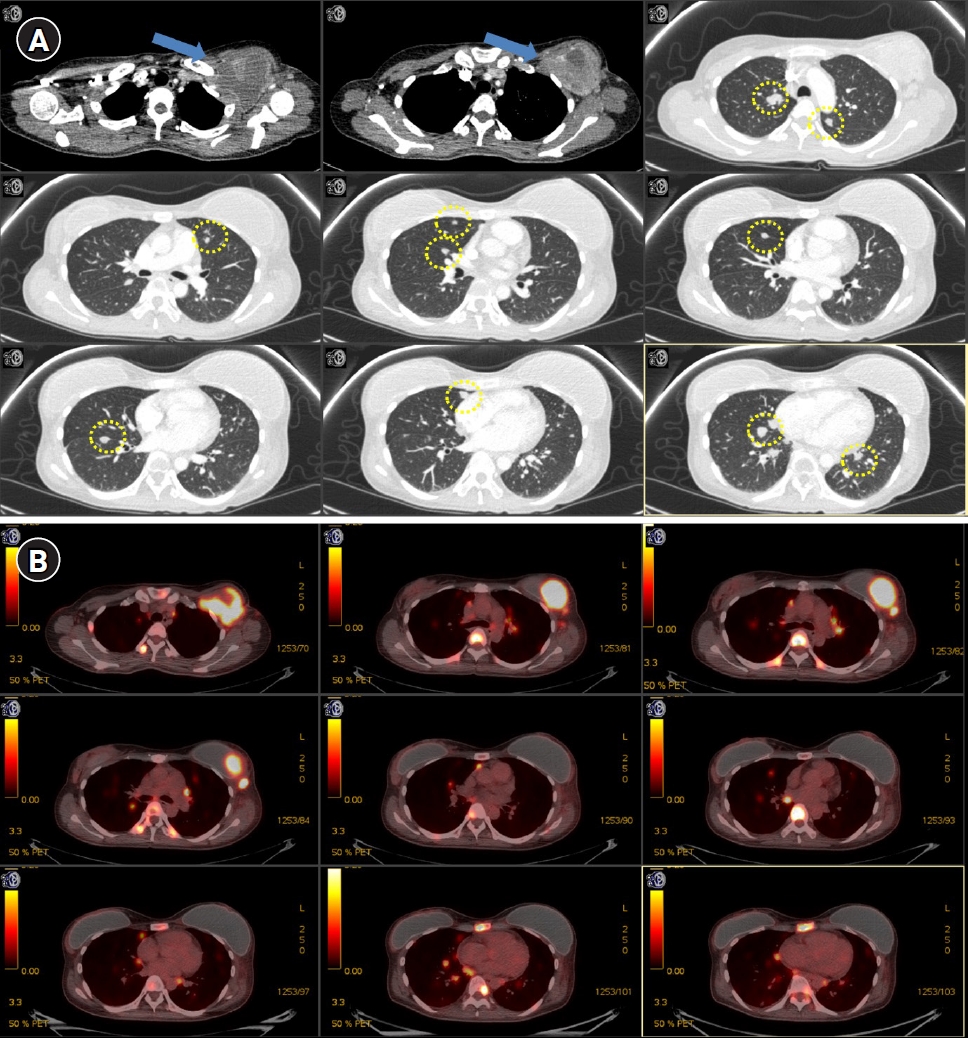
A 37-year-old woman was diagnosed with metastatic luminal B breast cancer (estrogen and progesterone receptor positive and HER2 positive) in April 2018. Metastatic lesions were found in the lungs (bilateral), bone (the spine, sternum, rib, both pelvic bone, and proximal femurs), and lymph nodes (left axillary level I, the left supraclavicular fossa, and the mediastinum). Positron emission tomography (PET) and chest computed tomography (CT) showed multiple metastatic lesions (Figs. 1, 2). She refused to receive any systemic therapy because she was afraid of toxicity caused by chemotherapy and hormone therapy. However, she agreed to receive palliative RT in order to relieve severe pain.
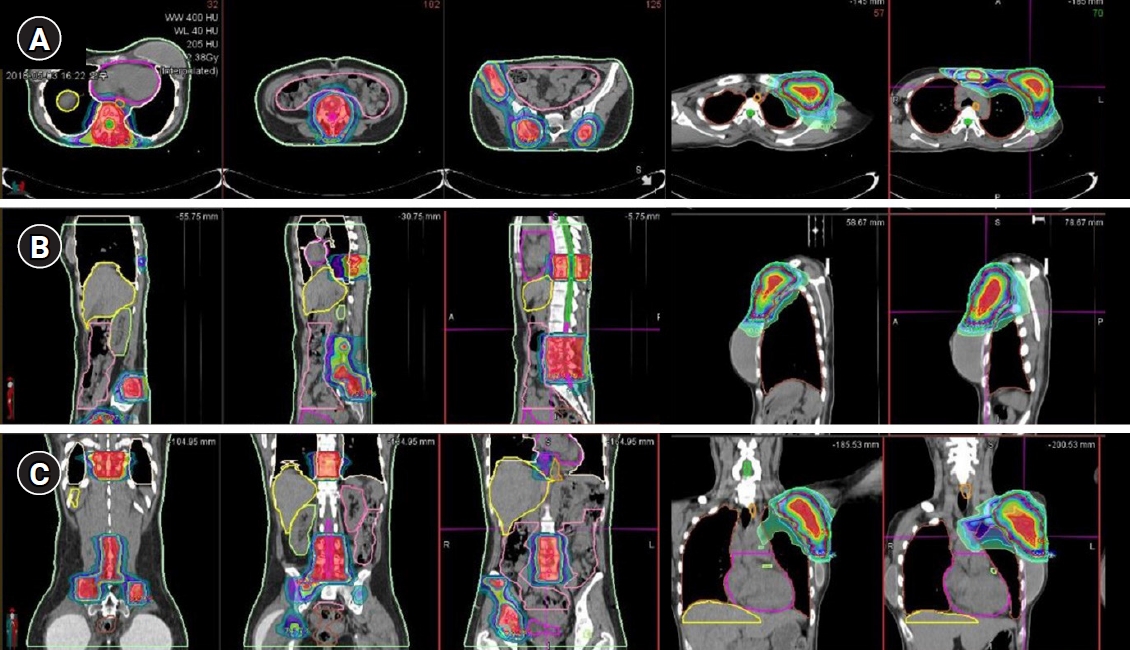
The patient received five fractions of IMRT for painful metastatic bone lesions. The patient had severe pain in the right pelvis, sacrum, and thoracic spine. The initial pain score using the visual analog scale (VAS) was 8. Simulation CT was performed using a custom-made immobilization device. The gross tumor volume (GTV) was contoured with the simulation CT fused with PET and diagnostic CT. The clinical target volume (CTV) was defined as the consideration of microscopic disease, and the planning target volume (PTV) was defined as an adequate margin for the CTV. A total dose of 22.5 Gy was delivered at 4.5 Gy to 100% of the PTV using IMRT to spare normal organs at risk, such as the heart, lung, bowel, and rectum. Linac-based volumetric modulated arc therapy was used for IMRT. After four fractions of RT of the thoracic spine and pelvic bone, the pain subsided (the VAS score reduced from 8 to 2), but there was new-onset pain in the left axilla and sternum (VAS score, 6). Additional RT to the axilla was planned, and the prescribed dose was 25 Gy in 5 Gy. Because the left axillary metastatic mass was large, a simultaneous integrated boost technique was used. The fractional dose of 6 Gy was prescribed to the “core” of axillary mass. The dose distribution for the thoracic spine, pelvic bones, sternum, and axillary mass are shown in Fig. 3. The patient did not have any severe toxicity during RT.
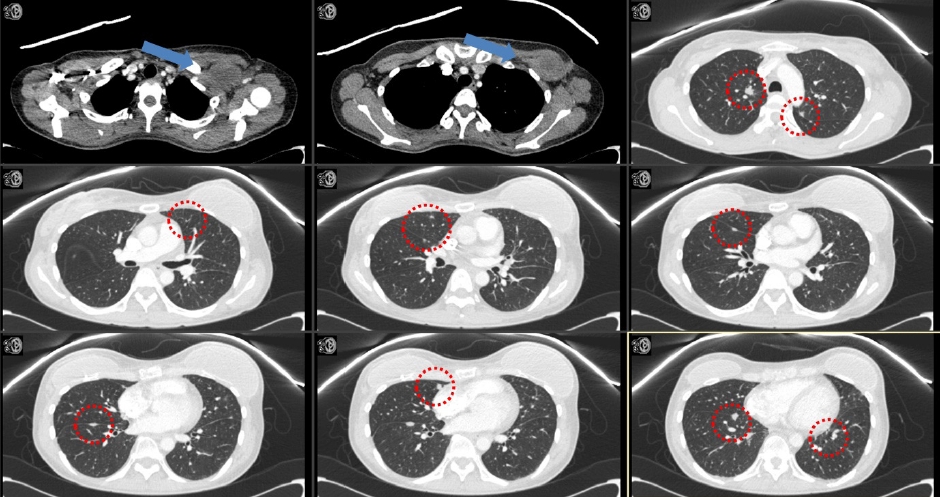
After 3 months of RT, the follow-up pulmonary CT revealed that a large mass involving the left anterior chest wall (irradiated lesion) and metastatic nodules in both lungs (unirradiated lesion) were markedly decreased in size (Fig. 4). Considering that RT was only administered to the chest wall mass, the decrease in the size of metastatic nodules in both lungs might be regarded as the abscopal effect. No other treatment was administered to either lung lesion. Severe pain in the thoracic spine, right pelvis, axilla, and sternum reduced from a VAS score of 8 to 0, from 8 to 2, from 8 to 2, and from 8 to 0, respectively.
As respect to lymphocyte count, total lymphocyte count (TLC) before RT was 3,300/mm3. TLC was decreased to 640 during RT, and TLC was recovered to 1,150 at 1-month follow-up after RT.
However, the patient did not want to receive any further treatment and refused to visit the hospital after 3 months of follow-up. The patient died of the disease 14 months after RT.
Discussion
The abscopal effect has been described as a rare phenomenon in clinical practice. This rarity is caused by the fact that conventional RT alone is inadequate to subvert the existing immunosuppression or tolerance characteristics of the microenvironment of an established tumor. However, the ability of RT to prime antitumor responses is likely to be key in not only inducing the abscopal effect but also obtaining therapeutic synergy with immunotherapies [4].
Considering Norman Coleman’s statement that “radiation is a different drug at different doses and fractionation schedules,” the dose and fractionation of RT might be linked to the abscopal effect. Vanpouille-Box et al. [4] provided a mechanistic explanation for the dose dependence of abscopal effects. A shorter course (≤3–5 fractions) with increasing dose size per fraction (≥6–8 Gy) was associated with activated intrinsic type I interferon (IFN-I) activation via the cGAS/STING pathway and may be the optimal choice of regimen to test abscopal responses. Although there are various dose and fractionation schedules for palliative RT (such as 8/1, 20/5, and 30/10), we believe that the shorter the course, the better the abscopal effect; shorter courses are also preferred for patients’ convenience.
Many groups have insisted that lymphocytes might play a key role in the abscopal effect, and the degree of RT-induced lymphopenia might influence the occurrence of the abscopal effect [5]. Short fractions lead to short periods of circulating lymphocyte depletion and relatively faster recovery. The Yonsei Group found that a large RT target volume was associated with RT-induced lymphopenia and poor survival [6]. Prevention of lymphopenia caused by RT might be needed to achieve the desired abscopal effect. Lambin et al. [5] proposed “lymphocyte-sparing RT” with the principle of “as low as reasonably achievable” in lymphocyte-rich regions in RT treatment planning. Recently, Sung et al. [7] developed a model for lymphocyte and RT, enabling physicians to predict the immunological consequences of different RT dose/fractions. This model could provide an explanation for understanding the abscopal effect.
Emerging data have shed light on the fact that lower tumor burden is associated with better response to immunotherapy [8]. Accordingly, inter-metastasis tumor heterogeneity could be a challenge for the abscopal effect as well as immunotherapy [9]. Weichselbaum, who first proposed the concept of oligometastasis in 1995 [10], suggested multi-site RT or RT to all sites because of the potential benefits of ablating non-immunogenic lesions [3]. Tumor microenvironment profiles are associated with response to immunotherapy. Recent studies have focused on immune checkpoint molecules, such as programmed death (PD)-1, PD-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which are associated with response to immunotherapy. Luke et al. [11] reported stereotactic body RT (SABR) outcomes based on local tumor response and tumor gene expression patterns, showing that SBRT increased the expression of innate and adaptive immune genes and decreased the expression of cell cycle and DNA repair genes. The limitation of our report is the lack of biopsy results. There was only an initial pathology report of a mastectomy specimen, and no post-RT pathology report. If there were both pre-RT and post-RT pathologies, additional analysis would be possible. According to changed policies of IRB in 2013, it was impossible to access the patient’s pathological records without the patient’s consent. At the time of writing this case report, the patient had already died and we could not get consent because we couldn’t contact her guardians. Recently, we initiated our phase II study (NCT04017897) to investigate whether immunotherapy plus RT exhibits increased antitumor activity in patients with melanoma [12]. Because we could not analyze various molecular profiles and tumor microenvironment due to absence of pathologic confirmation, we will check various molecular profiles and report changes in tumor microenvironment by obtaining peripheral blood sample and tumor tissue in the prospective study.
Several groups have provided preclinical and clinical evidence that low-dose radiation (doses below the threshold thought to kill cancer cells, e.g., 1–20 Gy total with <1–2 Gy daily dose) might convert the stroma by reducing transforming growth factor-beta (TGF-β) signaling and subsequently increasing the abscopal effect [13,14]. Low dose with high dose RT is currently underway in NCT02710253 at MD Anderson. Low-dose radiation has been used for decades (e.g., whole-lung RT 12–20 Gy for Ewing sarcoma) [15]. Some groups have suggested that irradiating parenchymal sites (e.g., the liver and lung) may be more associated with the abscopal effect because there are inherent differences in the microenvironment of various organs [16].
In summary, a meaningful abscopal effect is an infrequent phenomenon. Dose and fraction (conventional vs. hypofractionated vs. high-ablative dose), use of targeted image-guided techniques with a sharp fall-off of dosing (such as SABR), lymphocyte-sparing RT, tumor burden (oligometastases vs. polymetastases), targets (single site vs. multi-site), use of low-dose radiation, and irradiated sites (parenchymal vs. non-parenchymal) may affect the occurrence of abscopal effects. Furthermore, concomitant use of immunotherapy and the timing and sequence of RT in relation to immunotherapy can influence the abscopal effect and treatment outcomes. With emerging evidence of oligometastasis and the potential role of local ablative therapy, clinicians should be aware of the potential role of RT in immunomodulation.