 |
 |
AbstractPurposeThere has been limited work assessing the use of re-irradiation (re-RT) for local failure following stereotactic spinal radiosurgery (SSRS). We reviewed our institutional experience of conventionally-fractionated external beam radiation (cEBRT) for salvage therapy following SSRS local failure.
Materials and MethodsWe performed a retrospective review of 54 patients that underwent salvage conventional re-RT at previously SSRS-treated sites. Local control following re-RT was defined as the absence of progression at the treated site as determined by magnetic resonance imaging.
ResultsCompeting risk analysis for local failure was performed using a Fine-Gray model. The median follow-up time was 25 months and median overall survival (OS) was 16 months (95% confidence interval [CI], 10.8–24.9 months) following cEBRT re-RT. Multivariable Cox proportional-hazards analysis revealed Karnofsky performance score prior to re-RT (hazard ratio [HR] = 0.95; 95% CI, 0.93–0.98; p = 0.003) and time to local failure (HR = 0.97; 95% CI, 0.94–1.00; p = 0.04) were associated with longer OS, while male sex (HR = 3.92; 95% CI, 1.64–9.33; p = 0.002) was associated with shorter OS. Local control at 12 months was 81% (95% CI, 69.3–94.0). Competing risk multivariable regression revealed radioresistant tumors (subhazard ratio [subHR] = 0.36; 95% CI, 0.15–0.90; p = 0.028) and epidural disease (subHR = 0.31; 95% CI, 0.12–0.78; p =0.013) were associated with increased risk of local failure. At 12 months, 91% of patients maintained ambulatory function.
IntroductionEstimated to occur in 40%–50% of advanced cancers [1], osseous spinal metastases are associated with substantial morbidity including pain, vertebral column fractures, and neurologic compromise manifesting as incontinence, radiculopathy, quadriparesis, respiratory arrest, and/or ambulatory dysfunction [2-4]. Patient ambulation is central to functional independence and has been shown to be a critical factor associated with health-related quality of life (HRQoL) and overall prognosis [5]. While treatment of spinal metastases alone is rarely curative, the use of local radiation therapy (RT) with or without surgery has shown to provide significant benefits including prolongation of survival, preservation of ambulation, maintenance of neurologic functions, and reduction of pain [6]. Spine stereotactic radiosurgery (SSRS) has been established as a safe and effective treatment for spinal metastatic disease by providing precise targeting of tumor volume with higher biological effective doses (BED) while sparing sensitive adjacent structures [3,6-9]. In recent years, advances in technology have allowed SSRS to be increasingly utilized for upfront treatment of spine metastases in place of conventionally-fractionated external beam radiation (cEBRT) [10,11].
Though SSRS is thought to confer more durable disease control when compared with conventionally-fractionated RT, particularly for radioresistant histologies [6,9,12], 10%–20% of patients nevertheless develop local failure within 12 months following treatment [13-15]. As such, there has been growing interest in better characterizing salvage therapy options for these patients. While there have been several studies defining the role of salvage re-irradiation (re-RT) after conventionally-fractionated RT [3,16-18], evidence regarding the safety and efficacy of cEBRT for local failure following SSRS is limited. While repeat SSRS has been reported to confer roughly 80% 1-year local control (LC) rate [15], the efficacy of cEBRT following failure after SSRS is unknown. When compared with repeat SSRS, cEBRT for re-RT has several potential advantages including more widespread access and lower resource utilization. Additionally, SSRS may not be universally accessible to patients across different races, insurances, or geographical regions [19]. cEBRT also covers the epidural space more completely, which is advantageous given that the most common pattern of failure following SSRS is an epidural location [13,15]. In some patients, SSRS may be contraindicated due to spine instability, cord compression, or the number of vertebrae involved. cEBRT may provide superior disease coverage as a salvage option following SSRS in patients with greater extent of disease.
The goal of this study was to review our institutional experience with the use of cEBRT for local spinal relapse following SSRS with a focus on safety and efficacy. We also sought to determine prognostic variables that may influence the overall survival (OS) and LC of using cEBRT re-RT for spine metastases previously treated with SSRS.
Materials and Methods1. PatientsWe performed a retrospective review of 54 patients that underwent conventional re-RT following image-confirmed local progression at a spinal metastatic site that was previously treated with SSRS at a single large, comprehensive cancer center between 2005 and 2020. Each patient was carefully chosen for SSRS following multidisciplinary discussions of patient eligibility. Patients were commonly selected for SSRS for having good performance status, minimal vertebral level involvement, and limited additional metastases. Chart review was performed after obtaining the Institutional Review Board approval by the University of Texas MD Anderson Cancer Center (No. PA13-0218). Written informed consent from subjects in this retrospective chart review was not required.
SSRS and re-RT dose constraints for organs-at-risk were in agreement with previously published recommendations [20,21]. A minimum interval of 6 months was preferred between RT courses. Individualized decisions to re-irradiate earlier than 6 months were determined on a case-by-case bases after multidisciplinary review and discussions of the risks and benefits of re-treatment with the patient.
Patient age at diagnosis, age at spinal metastasis, number of metastases at re-RT, sex, race, Karnofsky performance score (KPS), pain scores, local therapies, therapy-related toxicities, vertebral column fractures, epidural disease at re-RT, paraspinal disease at re-RT, date of last follow-up, ambulatory status, and date of local failure were collected from the medical record. Solitary bone disease was defined as disease with a single bone metastasis without visceral organ metastases at the time of re-RT. Limited LC has been reported with use of cEBRT for radioresistant spine tumors [12,22]. To assess whether tumor radiosensitivity impacted patient response to spinal re-RT, patients were classified as having either radioresistant or radiosensitive metastases based upon the histology of the primary lesion. Based on our institutional practice, histologies that were considered radioresistant included renal cell carcinoma (RCC), urothelial carcinoma, melanoma, sarcoma, hepatocellular carcinoma (HCC), cholangiocarcinoma, colorectal adenocarcinoma, and non-small cell lung cancer [9]. Radiosensitive histologies included thyroid, breast, anal, prostate, and esophagogastric adenocarcinomas.
2. Latency to initial SSRS, local control and overall survivalLatency to SSRS was calculated from the time of spinal metastasis diagnosis to SSRS treatment. Local failure following SSRS was defined as radiographic progression of disease at the previously treated site. LC following re-RT was defined as the absence of progression of disease at the treated site as assessed on magnetic resonance imaging (MRI). Radiographic progression following both SSRS and re-RT was defined as an increase in tumor size at the treated site. LC and OS were estimated using the Kaplan-Meier method. OS were also estimated for patients with radioresistant disease that were treated with either 40 Gy or 30 Gy in 10 fractions using the log-rank method. Median follow-up time was estimated using the reverse Kaplan-Meier method. Predictors of LC and OS were determined using univariate and multivariable Cox proportional-hazards analysis. Univariate and multivariable competing risk regressions were also performed considering local progression and death as competing factors.
3. Evaluation of clinical characteristics and outcomes following SSRS and re-RTPatient RT-related acute toxicities, radiation-induced myelopathy or neuropathy, and frequency of vertebral column fractures (VCF) were determined following both SSRS and re-RT. SSRS and re-RT dose, fractionation, and technique were also abstracted and compared between groups. KPS was abstracted from patient charts at 1-, 3-, 6-, and 12-month following re-RT. Median KPS was calculated at each time point and adjusted to account for patients that were lost to follow-up or deceased at each time point. All patients were ambulatory prior to re-RT. When it occurred, date of loss of ambulatory status following re-RT was noted.
4. Statistical considerationsSignificance between patient and treatment characteristics was determined using a p-value threshold of ≤0.05. Normality and lognormality tests were performed to determine appropriate parametric and non-parametric tests for continuous variables. Categorical variables were assessed using χ2 or Fisher exact tests. Comparisons of OS and LC between groups were performed using both the Gehan-Breslow-Wilcoxon and log-rank methods. Competing risk analysis for local failure was performed using the Fine-Gray model. Subhazard ratios (subHR) from the Fine-Gray competing risk analysis describe the effect of the variables on the event of interest while accounting for competing events [23]. Statistical calculations were performed with Prism 9.1.2 (GraphPad Software, Boston, MA, USA), RStudio (RStudio Inc., Boston, MA, USA), and Stata 16.1 SE (StataCorp LLC, College Station, TX, USA).
Results1. Patients demographics, SSRS treatment, and disease characteristicsWe analyzed data from 54 patients that underwent conventional re-RT following image-confirmed local progression at a spinal metastatic site that was previously treated with SSRS. Forty-one of patients (76%) had radioresistant metastases and 13 (24%) had radiosensitive histologies. Patient demographics and disease characteristics are detailed in Table 1. Baseline patient characteristics were balanced among both radiosensitivity groups. Most patients were non-Hispanic White (65%) and male (61%). Prior to SSRS, the proportion of patients with a KPS ≥80 was 92% for radiosensitive histologies and 91% for radioresistant histologies (Table 2). A higher proportion of patients with radioresistant disease were treated with higher BED10 (p = 0.0001). Radioresistant patients also had nominally higher rates of prior surgery at the SSRS site (29%) compared to the radiosensitive group (8%), though this value was not statistically significant (p = 0.15). At the time of re-RT, most patients had epidural disease (80%) and multiple visceral metastases (83%), whereas a minority had paraspinal disease (39%).
2. SSRS-local failure and re-irradiation characteristicsDespite differences in SSRS treatment doses, there was no difference in the median time to local failure between the radioresistant (11.9 months; 95% confidence interval [CI], 12.5–21.5) and radiosensitive cohorts (8.6 months; 95% CI, 6.0–26.2; p = 0.54) (Table 3). Following local failure, patients underwent fractionated re-RT to the site of spinal metastases. The median time between SSRS and re-RT was 13.1 months (95% CI, 9.5–17.9) and did not differ among radiosensitivity groups (p = 0.982). Patients were retreated due to either progression at treated site without pain (74%, 40/54) or progression with pain (26%, 14/54). Additionally, most patients received re-RT more than 6 months after initial SSRS (83%). The techniques used were 2D/3D (68%) and intensity-modulated radiation therapy (IMRT, 32%). KPS prior to re-RT, radiation technique, salvage surgery, and radiation dose were not significantly different between radioresistant and radiosensitive groups. Collectively, 33% of patients received salvage surgery prior to re-RT. Two patients received systemic therapies concurrent with re-RT; one with nivolumab/ipilimumab and one with gemcitabine. Most patients received 30 Gy in 10 fractions (78%) or 40 Gy in 10 fractions (15%). All patients receiving 40 Gy were treated with IMRT utilizing a simultaneous integrated boost (SIB) technique that restricted the dose to the spinal cord to a maximum of 34 Gy [24]. Patients with radioresistant histologies were preferentially chosen for treatment with 40 Gy in 10 fractions using a SIB. Other RT dose-fractionation schemes included 30 Gy in 20 fractions (n = 1), 20 Gy in 5 fractions (n = 1), 33 Gy in 14 fractions (n = 1), and 20 Gy in 10 fractions (n = 1). For cEBRT after SSRS, a high priority was given to keeping the 110% isodose line off the spinal cord. Examples of 3D treatment plans for cEBRT after SSRS versus cEBRT alone can be visualized in Fig. 1.
Across all patients, the most common acute toxicities included fatigue (37%), pain (33%), and esophagitis/dysphagia (24%). There were no acute toxicities of grade 3 or higher reported. Grade 2 toxicities accounted for 26% of the re-RT toxicities. There were five patients that experienced VCF at the spinal site following re-RT (9%) and were not different between radiosensitive and radioresistant groups. Most VCF following salvage RT (4/5) were asymptomatic. Of patients that developed VCF, 60% (3/5) maintained local control for more than 15 months. All VCFs were believed to be attributable to disease progression. One patient with leiomyosarcoma developed grade 2 radiation-induced myelopathy. The patient was treated with 30 Gy in 10 fractions 17.9 months following SSRS, and developed myelopathy 4.7 months after re-RT. SSRS treatment provided a mean dose of 2,392 cGy and 788 cGy to the gross tumor volume (GTV) and spinal cord, respectively. Subsequent IMRT used a mean dose of 3,135 cGy and 791 cGy to GTV and spinal cord, respectively. This patient experienced weakness and pain bilaterally in the lower limbs, but did not severely impair ambulation. The patient was treated with steroids and did not develop local failure until 2 years following re-RT. There were no other cases of radiation-induced myelopathy or neuropathy in our cohort.
3. Survival and local control outcomesMedian follow-up time following cEBRT was 25 months (95% CI, 19.9–undefined) (Table 3). Rates of local failure were not significantly different among radioresistant and radiosensitive tumors (p = 0.19) or patients treated with 40 Gy versus 30 Gy (p = 0.503). LC for the entire cohort at 12 months was 81% (95% CI, 69.3–94.0) (Fig. 2A). Univariate analysis revealed age at diagnosis, age at spinal metastases, and KPS prior to SSRS were significantly associated with LC (Table 4). However, after multivariable analysis, only age at spinal metastases and KPS prior to SSRS remained significant. Competing risk regression multivariable analysis between OS and LC revealed radioresistant histology (subHR = 0.36; 95% CI, 0.15–0.90; p = 0.03) and the presence of epidural disease (subHR = 0.31; 95% CI, 0.12–0.78; p = 0.01) were associated with increased risk of local failure (Table 5).
The median OS from re-RT was 15.8 months (95% CI, 11.7–26.7) for the entire cohort (Fig. 2B). Univariate analysis revealed that age at diagnosis, radiosensitivity, sex, race, number of metastases, presence of epidural disease, pre-SSRS surgery, pre-SSRS KPS, number of non-contiguous SSRS-treated sites, BED10, or salvage surgery were not associated with OS (Table 4). KPS prior to re-RT (HR = 0.96; 95% CI, 0.93–0.99; p = 0.003), time to SSRS local failure (HR = 0.97; 95% CI, 0.94–0.996; p = 0.003), and presence of solitary bone disease (HR = 0.30; 95% CI, 0.10–0.87; p = 0.027) were significantly associated with OS. Male sex (HR = 1.90; 95% CI, 0.91–3.80; p = 0.09) was also not significant. Multivariable Cox proportional hazards analysis revealed KPS pre-re-RT (HR = 0.95; 95% CI, 0.93–0.98; p = 0.003) and time to local failure following SSRS (HR = 0.97; 95% CI, 0.94–1.00; p = 0.04) to be significantly associated with improved OS, while male sex (HR = 3.41; 95% CI, 1.46–8.34; p = 0.004) was associated with worse OS. Using the log-rank test, no difference was observed in OS (p = 0.886) between patients receiving 30 Gy versus 40 Gy.
4. KPS and ambulatory status after salvage RTMedian KPS was maintained between 70–80 up to 12 months after salvage RT (Fig. 3). Five patients developed ambulatory dysfunction (9%), a rate which did not differ between the radiosensitivity groups (p = 0.084) (Table 3). Of the five patients that developed ambulatory dysfunction, two patients developed neurologic compromise due to other metastatic sites, while three patients were limited in their ability to walk secondary to pain and musculoskeletal deconditioning. Ambulatory dysfunction in all five patients was not attributable to sequelae of radiation or progression at the treated site, but rather due to progression at other levels.
Discussion and ConclusionThe primary goal of re-RT is to improve patient QoL by controlling pain and preserving functional independence. The first application of SSRS was for the use of re-RT following cEBRT failure. Subsequent studies have assessed the outcomes and efficacy of SSRS following cEBRT failure and shown that SSRS re-RT can provide LC rates of 66%–93% with median OS of 10–21 months [3,16-18]. However, with the increasing use of SSRS as first-line RT for spine metastases, questions remain regarding how to manage local recurrence following SSRS. Several series have examined re-SSRS following local failure and have shown a 48% 1-year OS with minimal toxicity [15]. However, re-SSRS may not always be a preferred option for patients as SSRS may not be readily available at all centers, requires lengthy treatment planning with highly specialized dosimetry and physics teams, and additionally requires longer treatment times, which patients with painful spinal metastases may not be able to tolerate. Our experience with cEBRT following SSRS failure shows comparable efficacy to reports using re-SSRS following SSRS failure [15]. There were no grade 3–5 late toxicities reported, and despite one patient experiencing radiation-mediated myelopathy, this patient was able to maintain exceptional local control and was still alive at last follow up. Similarly, while 9% (n = 5) of patients developed VCF following re-RT, most durably maintained local control prior to progression.
One concern that treating physicians may have with using cEBRT following SSRS failure may be inadequate efficacy for radioresistant histologies [9,12]. In the current series, radiosensitivity grouping was not associated with either OS or LC in the multivariable model. However, the competing risk assessment between OS and local failure found radioresistant tumors to be associated with an increased risk of local failure. Despite this, there was no difference seen at in median KPS or in the rate of ambulatory dysfunction between the radiosensitive and radioresistant groups. We also assessed whether different cEBRT doses were associated with survival or LC. With 40 Gy in 10 fractions, concerns about high risks of toxicity may exist given that 30 Gy is typically delivered to the cord. While no such increased toxicity risk was observed, we also failed to observe differences in OS or LC between patients re-treated with 40 Gy and those treated with 30 Gy. A true dose-response relationship could not be robustly evaluated given the limited number of dose/fractionation schemes and subgroups sizes presented in the current study.
The only variables significantly associated with survival were KPS prior to re-RT, time to local failure following SSRS, and male sex. Performance status is a well-established predictor of OS in patients treated with RT bone metastases [25-27]. Furthermore, an accelerated rate of local failure is indicative of more aggressive disease and often worse prognosis. Male sex has been associated with decreased survival in a variety of cancers [28]. However, there have been mixed reports regarding the prognostic value of sex for OS in patients with spine metastases treated with radiation [26,27]. We assessed whether variability in sex as a prognostic marker could be confounded by tumor histology as men comprised most of the patients with RCC (10/13) and HCC (7/7) in the present study. However, in our univariate analysis, these tumor histologies did not associate with worse survival. It is possible that the prognostic significance of male sex could be confounded by other factors not explored in this study such as mutational burden and tumor size [15,18,29]. We observed a significant association of local failure with epidural disease, consistent with prior reports [13,15].
While we observed limited late toxicities and favorable outcomes, re-RT should be used in carefully selected patients with a full discussion of the potential risks and benefits in shared decision-making with the patient. The tolerance of organs-at-risk depends on various factors including age, treatment dose, fractionation pattern, organ type, tumor type, and time interval between re-RT [30]. It has been estimated that spinal cord recovery following RT after 6 months is 25%, and radiation earlier than 6 months can significantly increase the risk of myelopathy [20]. We generally recommend having a minimum interval of 6 months between radiation courses to mitigate the risk of toxicity. We do not recommend using re-RT if 3 months or less has lapsed since prior treatment and we generally exercise caution if the interval is between 3 and 6 months.
To our knowledge, this is the first study to assess the efficacy and safety of cEBRT following SSRS failure. There are inherent limitations to this retrospective study including selection and information bias. Additionally, we are limited in our ability to determine the impact of cEBRT re-RT on pain management and other QoL measures as these are not routinely documented before and after therapy. Given that many patients with spinal metastatic disease ultimately succumb, it is possible that the true rate of late cord toxicities from re-RT is higher than reported. Assessment of subclinical myelopathy was not possible given that formal organ level assessments were not performed. Furthermore, a comparison group was not included in this study, limiting our ability to make conclusions about this technique relative to others. The patient population in this study was heterogenous with a wide variety of antecedent treatments and different tumor histologies, which may have introduced confounding factors. We were also limited in our ability to detect prognostic factors for OS and LC in the setting of a limited number of events observed. Inclusion of more granular information regarding treatment volumes and a greater diversity of tumor histologies could provide more robust evaluation of prognostic factors associated with the efficacy of re-RT with cEBRT. Still, the strengths of this study include long patient follow-up using serial MRI assessment in a relatively large patient population allowing us to more readily determine tumor response to RT. We further attempted to include a variety of variables and adjust for known confounders with the use of multivariable and competing risk modeling.
In conclusion, our data show that re-RT with cEBRT for SSRS-treated metastases is feasible and associated with favorable local control, and that re-RT with conventional fractionation following local failure after SSRS may be safe and effective for patients. cEBRT re-RT was associated with comparable median OS and LC with patients treated with re-SSRS from historical controls and conferred minimal toxicities. For maximal safety, we recommend a latency of at least 6 months following SSRS before re-treatment.
NotesStatement of Ethics All patient information was de-identified in this study. Written informed consent from subjects in this retrospective chart review was not required. The study was approved by our institutional review board on human research, and was specifically reviewed and approved by the Institutional Review Board of University of Texas MD Anderson Cancer Center (No. PA13-0218). Funding This work was supported in part by Cancer Center Support (Core) from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center (No. P30 CA016672). The project was also supported by the Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship to Promote Diversity in Health-Related Research (No. F31-Diversity-1F31HL156500), the American Society of Hematology Minority Hematology Graduate Student Fellowship (MAF), and by the RSNA Research & Education Foundation through grant number RR2111 (BD). Author Contributions Conceptualization, Florez MA, De B, Chapman BV, Prayongrat A, Thomas JG, Ghia AJ. Investigation, Florez MA, De B, Chapman BV, Prayongrat A, Thomas JG. Formal analysis, Florez MA, De B. Writing of the original draft, Florez MA, De B, Chapman BV, Prayongrat A, Thomas JG, Beckham TH, Wang C, Yeboa DN, Bishop AJ, Briere T, Amini B, Li J, Tatsui CE, Rhines LD, Ghia AJ. Writing of the review and editing, Florez MA, De B, Chapman BV, Prayongrat A, Thomas JG, Beckham TH, Wang C, Yeboa DN, Bishop AJ, Briere T, Amini B, Li J, Tatsui CE, Rhines LD, Ghia AJ. Fig. 1.Comparison of three-dimensional treatment plans for cEBRT in a radiation-naïve patient (left) and cEBRT after SSRS (right). cEBRT, conventionally-fractionated external beam radiation; SSRS, spine stereotactic radiosurgery. 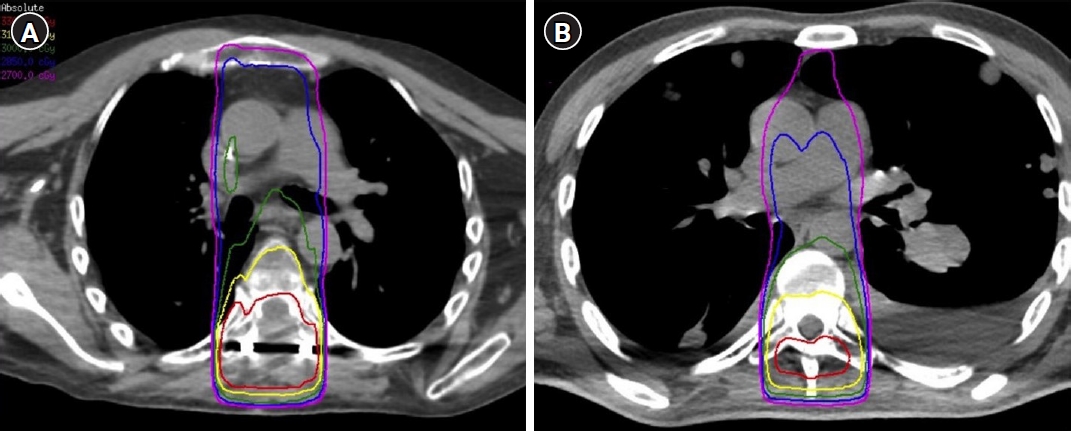
Fig. 2.(A) Local control and (B) overall survival Kaplan-Meir curves for included patients. re-RT, re-irradiation. 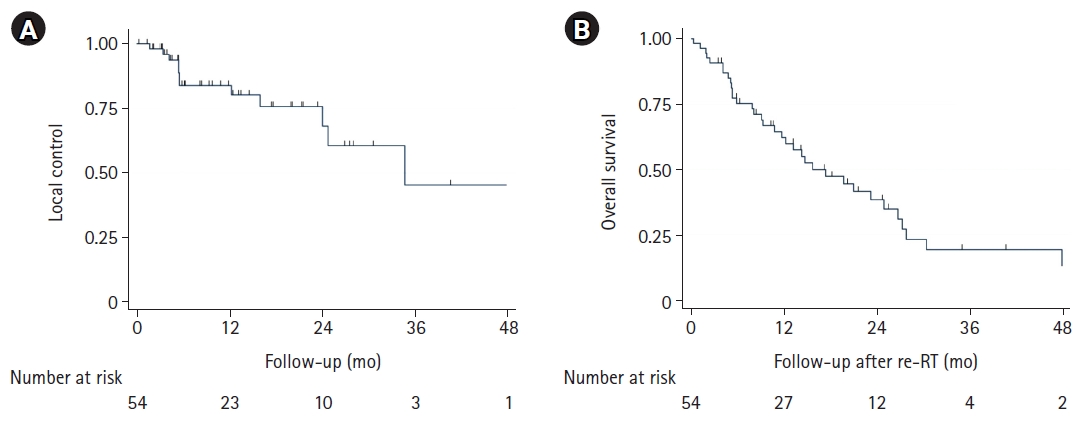
Fig. 3.Karnofsky performance status (KPS) at months following re-irradiation (re-RT) stratified by histology type. 
Table 1.Patient demographics and characteristics Values are presented as number of patients (%) or average ± standard deviation. NSCLC, non-small cell lung cancer; FTC, follicular thyroid carcinoma; PTC, papillary thyroid cancer; SCC, squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma. Statistical analysis was performed comparing radioresistant and radiosensitive histologies using appropriate tests. Table 2.Baseline characteristics of patients at the time of initial course of stereotactic radiosurgery Values are presented as average ± standard error of the mean or number of patients (%). SSRS, spine stereotactic radiosurgery; BED, biological effective dose; RT, radiotherapy; KPS, Karnofsky performance score. Statistical analysis was performed comparing radioresistant and radiosensitive histologies using appropriate tests. Table 3.Re-irradiation treatment characteristics and toxicities Values are presented as median (95% confidence interval) or number of patients (%). SSRS, spine stereotactic radiosurgery; LF, local failure; re-RT, re-irradiation; IMRT, intensity-modulated radiation therapy; PORT, post-operative radiation therapy; VCF, vertebral column fracture. Statistical analysis was performed comparing radioresistant and radiosensitive histologies using appropriate tests. Table 4.Mortality and local control hazard ratios Table 5.Competing risk regression analysis for local failure References3. Myrehaug S, Soliman H, Tseng C, Heyn C, Sahgal A. Re-irradiation of vertebral body metastases: treatment in the radiosurgery era. Clin Oncol (R Coll Radiol) 2018;30:85–92.
4. Wewel JT, O'Toole JE. Epidemiology of spinal cord and column tumors. Neurooncol Pract 2020;7(Suppl 1):i5–i9.
5. Barzilai O, McLaughlin L, Amato MK, et al. Predictors of quality of life improvement after surgery for metastatic tumors of the spine: prospective cohort study. Spine J 2018;18:1109–15.
6. Barzilai O, Laufer I, Yamada Y, et al. Integrating evidence-based medicine for treatment of spinal metastases into a decision framework: neurologic, oncologic, mechanicals stability, and systemic disease. J Clin Oncol 2017;35:2419–27.
7. Greco C, Pares O, Pimentel N, et al. Spinal metastases: from conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother 2015;20:454–63.
8. Zeng KL, Tseng CL, Soliman H, Weiss Y, Sahgal A, Myrehaug S. Stereotactic body radiotherapy (SBRT) for oligometastatic spine metastases: an overview. Front Oncol 2019;9:337.
9. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009;34(22 Suppl):S78–92.
10. Kotecha R, Dea N, Detsky JS, Sahgal A. Management of recurrent or progressive spinal metastases: reirradiation techniques and surgical principles. Neurooncol Pract 2020;7(Suppl 1):i45–i53.
11. Vellayappan BA, Chao ST, Foote M, et al. The evolution and rise of stereotactic body radiotherapy (SBRT) for spinal metastases. Expert Rev Anticancer Ther 2018;18:887–900.
12. Folkert MR, Bilsky MH, Tom AK, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys 2014;88:1085–91.
13. Bishop AJ, Tao R, Rebueno NC, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys 2015;92:1016–26.
14. Mehta N, Zavitsanos PJ, Moldovan K, et al. Local failure and vertebral body fracture risk using multifraction stereotactic body radiation therapy for spine metastases. Adv Radiat Oncol 2018;3:245–51.
15. Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys 2015;93:353–60.
16. Ito K, Ogawa H, Nakajima Y. Efficacy and toxicity of re-irradiation spine stereotactic body radiotherapy with respect to irradiation dose history. Jpn J Clin Oncol 2021;51:264–70.
17. Hashmi A, Guckenberger M, Kersh R, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: a multi-institutional outcome analysis. J Neurosurg Spine 2016;25:646–53.
18. Pontoriero A, Lillo S, Caravatta L, et al. Cumulative dose, toxicity, and outcomes of spinal metastases re-irradiation: systematic review on behalf of the re-irradiation working group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Strahlenther Onkol 2021;197:369–84.
19. Kim E, McClelland S, Jaboin JJ, Attia A. Disparities in patterns of conventional versus stereotactic body radiotherapy in the treatment of spine metastasis in the United States. J Palliat Care 2021;36:130–4.
20. Doi H, Tamari K, Oh RJ, Nieder C. New clinical data on human spinal cord re-irradiation tolerance. Strahlenther Onkol 2021;197:463–73.
21. Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2021;110:124–36.
22. Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine (Phila Pa 1976) 2009;34(22 Suppl):S48–57.
23. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391–400.
24. Farooqi A, Bishop AJ, Narang S, et al. Outcomes after hypofractionated dose-escalation using a simultaneous integrated boost technique for treatment of spine metastases not amenable to stereotactic radiosurgery. Pract Radiat Oncol 2019;9:e142.
25. Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–67.
26. Kubota H, Soejima T, Sulaiman NS, et al. Predicting the survival of patients with bone metastases treated with radiation therapy: a validation study of the Katagiri scoring system. Radiat Oncol 2019;14:13.
27. Westhoff PG, de Graeff A, Monninkhof EM, et al. An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys 2014;90:739–47.
28. Radkiewicz C, Johansson AL, Dickman PW, Lambe M, Edgren G. Sex differences in cancer risk and survival: a Swedish cohort study. Eur J Cancer 2017;84:130–40.
|
|
|||||||||||||||||||||||||||||||||||||||||

 |
 |