 |
 |
AbstractPurposeWe aimed to compare the oncological outcomes and toxicities of definitive proton beam therapy (PBT) and photon beam therapy in patients with limited-stage small cell lung cancer (LS-SCLC).
Materials and MethodsWe retrospectively reviewed 262 patients with newly diagnosed LS-SCLC who underwent definitive PBT (n = 20; proton group) or photon beam therapy (n = 242; photon group) with concurrent chemotherapy between January 2016 and February 2021 and compared overall survival (OS), progression-free survival (PFS), dose-volume parameters, and toxicities between the groups.
ResultsThe median follow-up duration was 24.5 months (range, 3.7 to 78.7). Baseline lung function was significantly worse and clinical target volume (CTV) was larger in the proton group (CTV: 296.6 vs. 215.3 mL; p = 0.080). The mean lung V10 was 37.7% ± 16.8% and 51.6% ± 24.5% in the proton and photon groups, respectively (p = 0.002). Two-year OS and PFS rates were 57.2% and 35.7% in the proton group and 65.3% and 40.8% in the photon group, respectively (p = 0.542 and 0.748, respectively). Grade ≥2 radiation pneumonitis and esophagitis occurred in 5 (25.0%) and 7 (35.0%) PBT-treated patients and 66 (27.3%) and 40 (16.5%) photon beam therapy-treated patients, respectively (p = 0.826 and 0.062, respectively).
ConclusionAlthough the proton group had poorer lung function and a larger CTV than that in the photon group, both groups exhibited comparable treatment outcomes and radiation-related toxicities in LS-SCLC. PBT may be a valuable therapeutic modality in patients with poor pulmonary function or extensive disease burden owing to its lung-sparing ability.
IntroductionAssessing pulmonary function is essential before determining whether thoracic surgery or definitive radiation therapy (RT) is necessary [1]. Poor baseline lung function is often considered a contraindication to definitive RT for lung cancer [1]. Radiation pneumonitis is one of the most important dose-limiting factors of RT in patients with thoracic cancer [1]. It is the most frequent toxicity following concurrent chemoradiotherapy (CCRT), with decreased respiratory function and reduced quality of life [2-5]. Worse pulmonary function at treatment may adversely affect lung complications or survival outcomes following definitive RT [6-8].
Proton beam therapy (PBT) can reduce radiation exposure to neighboring normal tissues due to its “Bragg peak” property [9]. The ability of PBT to reduce doses to these normal organs-at-risk (OARs) has been demonstrated in locally advanced non-small cell lung cancer, and dosimetric advancements have been reported in comparison with photon beam therapy [10-12]. The dosimetric benefits of PBT can result in reduced toxicity and even improved survival rates [13]. As CCRT is the mainstay of treatment for limited-stage small cell lung cancer (LS-SCLC), it can increase the risk of esophagitis and pulmonary toxicity, in part because of the anatomic proximity of the large target volume and centrally located disease to critical OARs [14,15]. However, evidence on the efficacy and safety of PBT compared to photon beam therapy in patients with LS-SCLC is scarce. Herein, we conducted a study to determine whether PBT is effective despite poor lung function compared to photon beam therapy in patients with LS-SCLC.
Materials and Methods1. Study design and patientsThis retrospective study was conducted at a single tertiary institution. The eligibility criteria were as follows: histologically diagnosed SCLC, no history of prior therapy for the targeted lesion, and underwent RT between January 2016 and February 2021.
After obtaining approval from the Institutional Review Board of Samsung Medical Center (No. 2022-10-108-001), we identified 511 patients who underwent RT for SCLC between January 2016 and February 2021. A total of 249 patients were excluded because of combined histology (n = 27); extensive stage (n = 12); treatment of salvage, adjuvant, or palliative therapy (n = 188); or RT alone (n = 8); and incomplete treatment (n = 14). Ultimately, we retrospectively reviewed the medical records of 262 patients; 20 patients had undergone PBT (proton group), while 242 had received photon beam therapy (photon group). A consort diagram is shown in Fig. 1. The requirement for informed consent was waived due to the retrospective nature of this study.
2. Treatment planMost chemotherapy regimens were comprised of etoposide and cisplatin administered via intravenous infusion. Based on our previous findings, CCRT was mostly initiated on the third cycle (n = 223, 85.1%), followed by the first cycle of chemotherapy [16]. Consolidative durvalumab was administered every 3 weeks when prescribed [17].
Prior to RT, each patient underwent a four-dimensional simulation computed tomography (CT). Based on all available clinical information, the gross tumor volume (GTV) was delineated on CT images from 10 breathing phases, covering all phases of the breathing cycle. The internal target volume (ITV) was generated from the GTV of each CT image. The clinical target volume (CTV) was defined by expanding up to a 0.5–0.7 cm margin from the ITV and was modified according to the adjacent organs. In cases where RT was administered after two cycles of chemotherapy, the CTV was modified to reflect primary tumor shrinkage, considering the post-chemotherapy chest CT images [16]. However, despite the significant response, the initially involved lymph node stations were included within the CTV [16]. An additional margin of 5 mm was applied to the CTV to generate the planning target volume. For the prescribed dose, a biologically effective dose was calculated using the standard linear-quadratic model with an α/β of 10 Gy for SCLC, a commonly used value. A median total dose of 52.5 Gy (range, 52.5 to 61.6) was prescribed, with a fractional dose of 2.1 Gy (range, 2.0 to 2.2), as reported previously [16]. The criteria for selecting PBT at our institution are poor pulmonary function at baseline or underlying pulmonary diseases, such as pulmonary fibrosis or chronic obstructive pulmonary disease. The relative biological effectiveness (RBE) of PBT was considered a fixed value of 1.1.
Prophylactic cranial irradiation (PCI) was administered to patients who achieved a complete or very good partial response after CCRT [16]. The main dose scheme for PCI was 25 Gy in 10 fractions.
3. Endpoints and statistical analysisThe primary endpoints were to compare patients’ characteristics, including pulmonary function and toxicities, between the two groups. The secondary endpoints were overall survival (OS) and progression-free survival (PFS). The duration of OS was calculated from the start date of chemotherapy to the date of death or last follow-up. Likewise, PFS duration was calculated from the start date of chemotherapy to the date of progression or the last follow-up. Dosimetric parameters for the target volume or normal organs were analyzed using dose-volume histograms. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 5.0).
Differences in continuous variables between the two groups were analyzed using Student t-test or the Mann-Whitney U test. Chi-squared or Fisher exact test was used to evaluate differences in categorical variables between the two groups. OS and PFS were estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariate analyses of OS and PFS were performed using Cox regression analysis. The RT modality and factors (p < 0.2 in the univariate analysis) were further assessed using multivariate analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS Statistics (version 27.0; IBM Inc., Armonk, NY, USA).
Results1. Patients' characteristics
Table 1 describes the patients’ baseline characteristics according to the RT modality. Approximately 75% of the patients in the photon group underwent intensity-modulated RT. The proton group comprised more patients aged ≥65 years than in the photon group (70.0% vs. 49.2%, respectively). Regarding baseline lung function, PBT-treated patients had worse values in terms of forced expiratory volume in one second, i.e., <50% of the predicted value (20.0 vs. 2.9; p = 0.012), and diffusing capacity for carbon monoxide (DLCO), i.e., < 60% of the predicted value (45.0 vs. 16.1; p = 0.011), than in patients who received photon beam therapy. There was no difference in the use of consolidative durvalumab between the two groups (19.8% vs. 10.0%; p = 0.383).
2. Dosimetric parametersThe proton group had a larger mean CTV than the photon group (296.6 vs. 215.3 mL; p = 0.080). The dosimetric parameters are shown in Fig. 2. The mean lung V10 was 37.7% ± 16.8% and 51.6% ± 24.5% in the proton and photon groups, respectively (p = 0.002). The mean lung dose in the proton group was numerically lower than that in the photon group, but it was not statistically different (857.3 vs. 983.1 cGy; p = 0.343). Furthermore, the proton group had a significantly lower mean heart dose than that in the photon group (651.5 vs. 938.4 cGy; p = 0.004). Additionally, we found that the mean esophagus V45 was 12.6% ± 18.7% and 10.9% ± 14.1% in the proton and photon groups, respectively (p = 0.697).
3. Clinical outcomesConsidering the total cohort, the median follow-up was 24.5 months (range, 3.7 to 78.7); 21.9 and 24.6 months in the proton and photon groups, respectively. Fig. 3 shows a comparison of OS and PFS between the two groups. The 2-year OS and PFS rates were 57.2% and 35.7%, respectively, in the proton group and 65.3% and 40.8%, respectively, in the photon group (p = 0.542 and 0.748, respectively); however, there were no statistically significant differences between the two groups.
Table 2 shows the univariate and multivariate models of OS. Univariate analysis revealed no statistically significant differences between RT modalities (p = 0.514). Multivariate analysis revealed that RT modality was not associated with OS (hazard ratio [HR] = 1.885; 95% confidence interval [CI], 0.878–4.050; p = 0.104), although DLCO, stage, PCI, and durvalumab were significant prognostic factors for OS.
Additionally, Table 3 shows the univariate and multivariate analyses of PFS. According to the Cox regression analysis, RT modality was not associated with PFS (HR = 1.064; 95% CI, 0.580–1.950; p = 0.841). Stage and PCI were demonstrated as significant prognostic factors for PFS.
4. Safety
Table 4 summarizes the treatment-related complications according to the RT modality. Grade ≥2 radiation pneumonitis was observed in five (25.0%) PBT-treated patients, and 66 (27.3%) patients who received photon beam therapy (p = 0.826). Furthermore, the proton group had a higher incidence of grade ≥2 radiation esophagitis than the photon group (35.0% vs. 16.5%; p = 0.062). Additionally, the rate of grade 2 or higher radiation dermatitis was not different between the two groups (5.0% vs. 1.2%; p = 0.274). None of the patients in either group experienced grade ≥4 toxicities.
Discussion and ConclusionThis study compared the clinical efficacy and safety of PBT and photon beam therapy in patients with LS-SCLC. Although PBT-treated patients had relatively worse lung function and a larger target volume than those who received photon beam therapy, the oncological outcomes and treatment-related toxicities did not significantly differ between the two groups.
In this study, neither OS nor PFS differed significantly between the two groups (p = 0.542 and 0.748, respectively). Specifically, the proton and photon groups demonstrated 5-year OS rates of 43.6% and 46.6%, respectively, which are consistent with previously reported rates [16,18]. The addition of thoracic RT has been shown to improve the survival of patients with LS-SCLC [18-20]. Previous meta-analyses that included more than 2,000 patients revealed that thoracic radiation for LS-SCLC could yield a 5% to 7% improvement in the 2-year OS compared to that in chemotherapy alone [19,20]. Furthermore, a more recent series has reported a 5-year OS rate exceeding 30%, approaching the outcomes of locally advanced NSCLC at a similar stage [18].
Although the rate of grade ≥2 esophagitis was higher in the proton group (35.0% vs. 16.5%; p = 0.062), PBT showed similar toxicity profiles compared to that in photon beam therapy. The higher rate of grade ≥2 esophagitis in the proton group might be caused by the higher dose to the esophagus as shown in the maximum dose of the esophagus and the results of esophagus V45. We also analyzed the shortest distances between CTV and esophagus in the total cohort. As a result, we identified that the mean distance was 4.7 ± 13.6 mm and 6.1 ± 11.7 mm in the proton and photon groups, respectively (p = 0.661). This anatomical relationship might have affected the results of esophagitis. PBT has been increasingly used as a definitive treatment for patients with LS-SCLC [14,21]. Colaco et al. [21] reported the results of six patients who received PBT with cisplatin/etoposide chemotherapy. Except for one patient, all patients received concurrent treatment, and only one received daily RT (60–66 Gy [RBE]). None of the patients experienced grade ≥3 toxicities. The largest prospective observational study involving 30 patients was recently reported [14]. All the patients received platinum and etoposide concurrently with PBT. Twelve patients received twice-daily PBT (45 Gy [RBE]), and the remainder underwent daily treatment (59.4–66.6 Gy [RBE]). Dosimetric analyses revealed substantially lower heart dose parameters with PBT, along with reductions in lung V5 and mean lung doses (not V20). Grade 3 toxicities were limited to one case of anorexia (daily treatment) and one case each of pneumonitis and pericardial effusion (twice-daily treatment). We consistently observed that PBT-treated patients had substantially lower lung V10 and mean heart dose values than in patients who received photon beam therapy. The findings of our study and those reported previously suggest that PBT may be tolerated and appropriate for limiting normal tissue toxicity, such as in normal lung tissues, because of its unique depth-dose curve with a Bragg peak [22,23]. Furthermore, given that patients with LS-SCLC can develop locoregional recurrence after the initial CCRT, PBT may allow safe re-irradiation [24].
Based on clinical trial data, the preferred first-line systemic treatment for extensive-stage SCLC involves durvalumab in combination with etoposide and cisplatin (or carboplatin), followed by maintenance durvalumab [25-27]. However, there is limited evidence supporting the use of immunotherapy in patients with LS-SCLC. A recent phase II study demonstrated the promising clinical efficacy of consolidative durvalumab in combination with CCRT for LS-SCLC [17]. With a median follow-up duration of 26.6 months, the 2-year OS rate was 67.8%. These findings suggest that durvalumab with CCRT may be clinically relevant, affording improved survival outcomes compared to the historical control treatment in patients with LS-SCLC. In this study, durvalumab was identified as a significant prognostic factor for OS in the multivariate analysis. Furthermore, it has been reported that proton or carbon ion may stimulate the immune system to a greater degree than photons [28,29].
Intracranial metastases have been found to occur in more than 50% of patients with SCLC. According to randomized studies, PCI can effectively decrease the incidence of brain metastases; however, most individual studies lack sufficient power to demonstrate a meaningful survival benefit [30]. However, a meta-analysis of available randomized trials reported a 5.4% increase in the 3-year OS of PCI-treated patients, from 15.3% in the control group to 20.7% in the PCI group [31]. The observed advantages were similar between the patients with LS and those with extensive-stage SCLC. Similarly, in this study, we identified PCI as a significant prognostic factor for OS. Accordingly, PCI can be recommended for patients with LS-SCLC. In contrast, shared decision-making is recommended for patients aged ≥70 years with a risk of neurocognitive impairment [17,32].
This study has several limitations. First, the potential for selection bias due to the retrospective nature of the study cannot be excluded. Second, the clinical outcomes may have been distorted due to the insufficient sample size and single-center design. Finally, the oncological outcomes may have been overestimated because of the short follow-up period. Despite these limitations, to the best of our knowledge, this study is the first to compare proton and photon therapies as curative RT modalities in patients with newly diagnosed LS-SCLC; hence, it is highly relevant.
In conclusion, despite poor pulmonary function and large target coverage, the PBT group did not differ from the photon group in radiation pneumonitis. Moreover, none of the patients experienced grade ≥4 toxicities. Thus, our findings suggest that PBT could be a feasible definitive treatment option, especially for patients with a high risk of pulmonary toxicity or who are expected to undergo extensive irradiation volume. Further large-scale studies, including prospective randomized controlled trials with long-term follow-ups, are required to validate our results.
NotesStatement of Ethics This study was approved by the Institutional Review Board of Samsung Medical Center (No. 2022-10-108-001). The study was performed in accordance with the Declaration of Helsinki. Author Contributions Conceptualization, Noh JM. Investigation and methodology, Seo SH, Noh JM. Project administration, all authors. Supervision, Noh JM. Writing of the original draft, Seo SH. Writing of the review and editing, Noh JM. Software, Seo SH, Noh JM. Validation, Seo SH, Noh JM. Formal analysis, Seo SH, Noh JM. Data curation, Seo SH, Noh JM. Visualization, Seo SH, Noh JM. All the authors have proofread the final version. Fig. 1.Consort diagram. RT, radiation therapy; SCLC, small cell lung cancer; CCRT, concurrent chemoradiotherapy; LS, limited-stage. 
Fig. 2.Comparison of dosimetric parameters of lung and other organs at risk between the proton group (red color) and photon group (blue color). Data are presented as the mean with standard deviation. Vx, volume receiving x% of the prescription dose; Dmean, mean dose; Dmax, maximum dose. 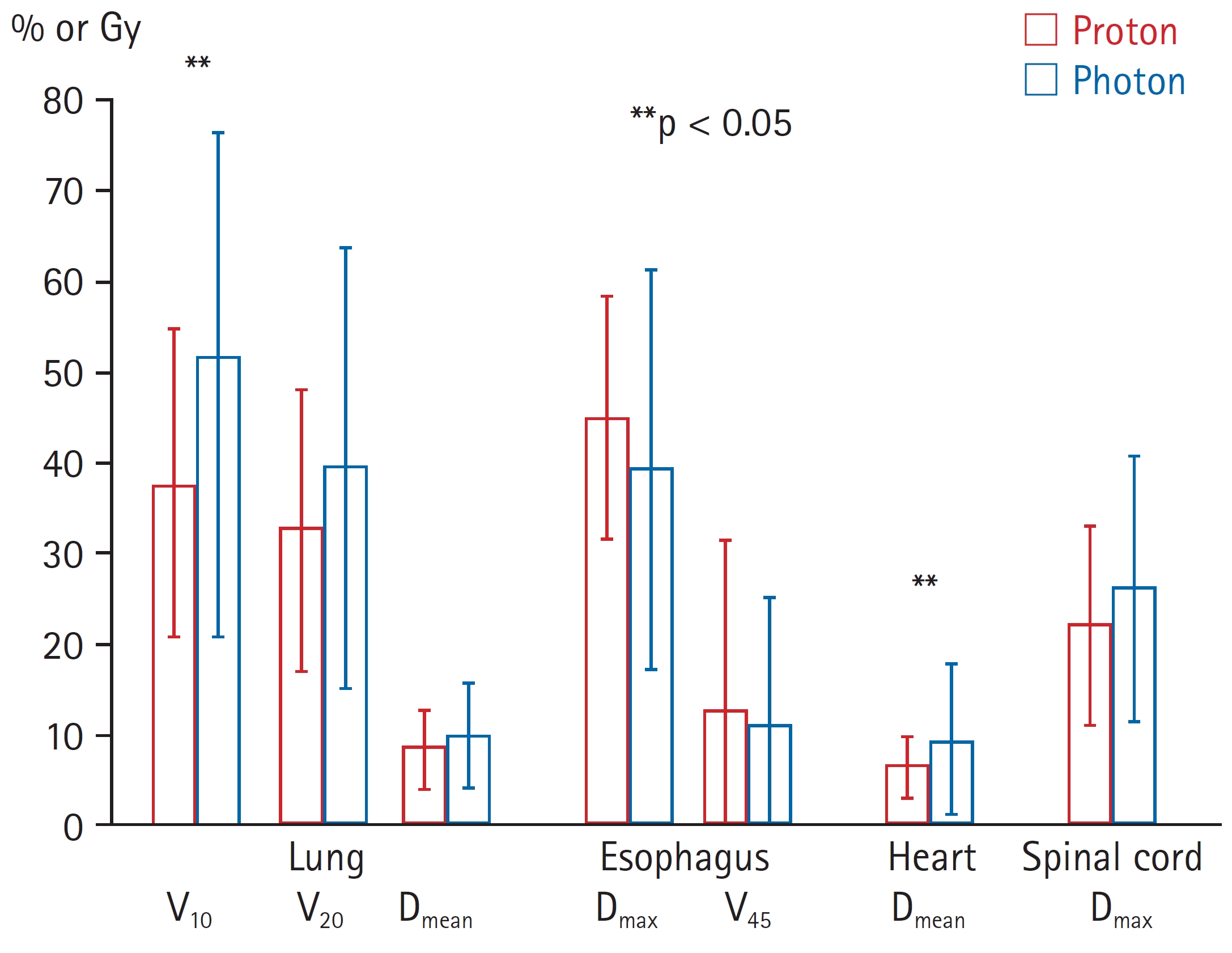
Fig. 3.(A) Overall survival and (B) progression-free survival between the proton group (red line) and photon group (blue line). 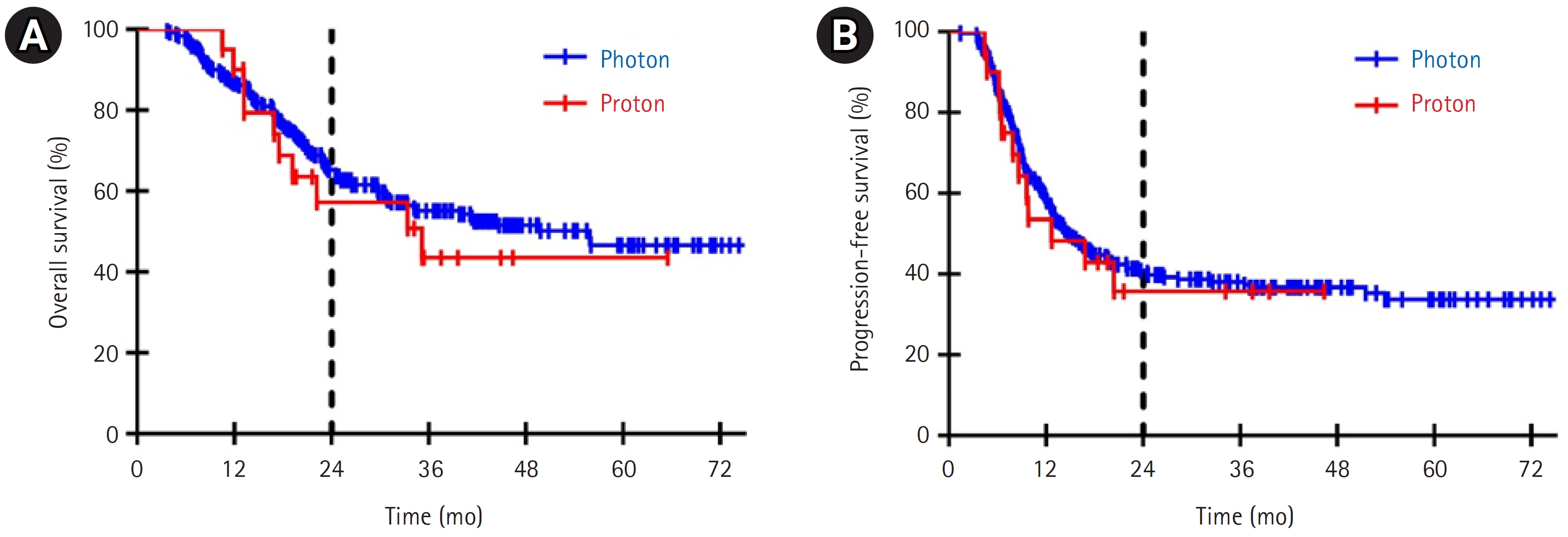
Table 1.Baseline characteristics according to radiation modality
Table 2.Prognostic factors for overall survival (OS) in univariate and multivariate analyses
RT, radiation therapy; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity for carbon monoxide; TTF1, thyroid transcription factor-1; CTV, clinical target volume; LDH, lactate dehydrogenase; PCI, prophylactic cranial irradiation; SER, time between the start of chemotherapy and the end of radiation therapy; HR, hazard ratio; CI, confidence interval. Table 3.Prognostic factors for progression-free survival (PFS) in univariate and multivariate analyses
RT, radiation therapy; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity for carbon monoxide; TTF1, thyroid transcription factor-1; CTV, clinical target volume; LDH, lactate dehydrogenase; PCI, prophylactic cranial irradiation; SER, time between the start of chemotherapy and the end of radiation therapy; HR, hazard ratio; CI, confidence interval. Table 4.Treatment-related toxicities according to radiation modality References1. Wang J, Cao J, Yuan S, et al. Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys 2013;85:798–804.
2. Kim M, Lee J, Ha B, Lee R, Lee KJ, Suh HS. Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer. Radiat Oncol J 2011;29:181–90.
3. Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol 2003;67:275–83.
4. Abratt RP, Morgan GW, Silvestri G, Willcox P. Pulmonary complications of radiation therapy. Clin Chest Med 2004;25:167–77.
5. Torre-Bouscoulet L, Munoz-Montano WR, Martinez-Briseno D, et al. Abnormal pulmonary function tests predict the development of radiation-induced pneumonitis in advanced non-small cell lung Cancer. Respir Res 2018;19:72.
6. Zhou Y, Yan T, Zhou X, et al. Acute severe radiation pneumonitis among non-small cell lung cancer (NSCLC) patients with moderate pulmonary dysfunction receiving definitive concurrent chemoradiotherapy: impact of pre-treatment pulmonary function parameters. Strahlenther Onkol 2020;196:505–14.
7. Semrau S, Klautke G, Fietkau R. Baseline cardiopulmonary function as an independent prognostic factor for survival of inoperable non-small-cell lung cancer after concurrent chemoradiotherapy: a single-center analysis of 161 cases. Int J Radiat Oncol Biol Phys 2011;79:96–104.
8. Lopez Guerra JL, Gomez DR, Zhuang Y, et al. Changes in pulmonary function after three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, or proton beam therapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e537–43.
10. Simone CB, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014;20:427–32.
11. Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087–96.
12. Nichols RC, Huh SN, Henderson RH, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage iii non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer 2011;12:252–7.
13. Higgins KA, O'Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:128–37.
14. Rwigema JM, Verma V, Lin L, et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer 2017;123:4244–51.
15. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
16. Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol 2013;24:2088–92.
17. Park S, Noh JM, Choi YL, et al. Durvalumab with chemoradiotherapy for limited-stage small-cell lung cancer. Eur J Cancer 2022;169:42–53.
18. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116–25.
19. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
20. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890–5.
21. Colaco RJ, Huh S, Nichols RC, et al. Dosimetric rationale and early experience at UFPTI of thoracic proton therapy and chemotherapy in limited-stage small cell lung cancer. Acta Oncol 2013;52:506–13.
22. Han Y. Current status of proton therapy techniques for lung cancer. Radiat Oncol J 2019;37:232–48.
23. Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2016;95:505–16.
24. Verma V, Rwigema JM, Malyapa RS, Regine WF, Simone CB. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol 2017;125:21–30.
25. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39.
26. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51–65.
27. Mathieu L, Shah S, Pai-Scherf L, et al. FDA approval summary: atezolizumab and durvalumab in combination with platinum-based chemotherapy in extensive stage small cell lung cancer. Oncologist 2021;26:433–8.
28. Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys 2016;95:120–30.
29. Shimokawa T, Ma L, Ando K, Sato K, Imai T. The future of combining carbon-ion radiotherapy with immunotherapy: evidence and progress in mouse models. Int J Part Ther 2016;3:61–70.
30. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183–90.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |