 |
 |
AbstractPurposeTo evaluate the clinical outcome of high-dose-rate (HDR) interstitial brachytherapy (IBT) in patients with oral cavity cancer.
Materials and MethodsSixteen patients with oral cavity cancer treated with HDR remote-control afterloading brachytherapy using 192Ir between 2001 and 2013 were analyzed retrospectively. Brachytherapy was administered in 11 patients as the primary treatment and in five patients as salvage treatment for recurrence after the initial surgery. In 12 patients, external beam radiotherapy (50-55 Gy/25 fractions) was combined with IBT of 21 Gy/7 fractions. In addition, IBT was administered as the sole treatment in three patients with a total dose of 50 Gy/10 fractions and as postoperative adjuvant treatment in one patient with a total of 35 Gy/7 fractions.
ResultsThe 5-year overall survival of the entire group was 70%. The actuarial local control rate after 3 years was 84%. All five recurrent cases after initial surgery were successfully salvaged using IBT ± external beam radiotherapy. Two patients developed local recurrence at 3 and 5 months, respectively, after IBT. The acute complications were acceptable (≤grade 2). Three patients developed major late complications, such as radio-osteonecrosis, in which one patient was treated by conservative therapy and two required surgical intervention.
IntroductionBrachytherapy is the use of radionuclide sources to treat malignancies by positioning the radiation source near or within the tumor. Brachytherapy results in a better dose distribution than external beam radiotherapy (ERT) because of the sharp dose fall-off in the surrounding normal tissues. Because adjacent normal tissues, such as the salivary glands, mandible, and mastication muscles, are exposed to radiation during ERT for oral cavity (OC) cancer, interstitial brachytherapy (IBT) can be used as a primary or supplemental treatment to improve local control without irradiating normal tissue. Low-dose-rate (LDR) IBT has long been used for head and neck cancers arising from the lip, tongue, floor of the mouth and buccal mucosa. In recent years, high-dose-rate (HDR) remote-control afterloading brachytherapy has become more popular over LDR IBT with several potential advantages [1], such as the lack of radiation exposure to medical personnel, a shorter patient immobilization time, more accurate treatment planning and delivery and treatment on an outpatient basis. Presently, HDR IBT using a three-dimensional (3D)-planning system facilitates the optimization of dose distribution based on dose-volume histograms [2]. The application of HDR IBT has been extended to many sites and has been used in the treatment of head and neck cancer [3], as well as gynecological, breast and prostate cancers [4,5]. Particularly, in OC cancer, the HDR IBT technique has been proven to demonstrate high tumor control and usefulness [6].
In the present study, we retrospectively evaluated the treatment outcomes of HDR IBT in patients with squamous cell carcinoma of the OC, along with a literature review.
Materials and Methods1. Patient characteristicsSixteen patients with squamous cell carcinoma of the OC treated with HDR IBT between 2001 and 2013 were reviewed retrospectively. The indications for HDR IBT were pathologically proven oral cavity cancer with no distant metastasis and in cases of medically inoperable or refusal of surgery. Eleven patients received IBT as a part of their initial treatment, and five patients received IBT as salvage treatment for recurrence after the initial surgery. In five recurrent cases who had tongue cancers as the primary, three patients had recurrence in the remaining portion of the tongue, and two had recurrence in the floor of the mouth after partial glossectomy. The patient characteristics are shown in Table 1. If patients had clinically positive nodes or high risks of occult metastases, the regional lymphatics were treated with ERT-3D conformal (until March 2007) or intensity-modulated radiotherapy (IMRT).
2. Implantation techniqueWhen combined with ERT, the implant procedure was performed 2-3 weeks after completion of ERT. During this time, supposedly most of the acute adverse reactions caused by ERT are resolved. All of the patients were hospitalized for the entire duration of HDR IBT. Before implantation was performed, the radiation oncologist defined the preliminary target volume using clinical examination and imaging studies in advance. The target for interstitial implants is defined as the residual tumor after ERT. In cases of complete response after ERT, residual induration or a previous tumor bed was the target for implantation. When IBT was performed as the sole treatment, the gross tumor was the target. Under general anesthesia, the target area is defined by bimanual palpation of the tumor bed, and hollow thin-wall stainless steel stylets (Trocar (part number 110.117) and Obturator (part number 110.171); Nucletron, Veenendaal, The Netherlands) were inserted through the ipsilateral submental and submandibular triangular region into the target. The tips of the stylets were brought through to the dorsum of the tongue. Usually, each stylet was placed approximately 10 mm apart and parallel to each other. Peripheral stylets should be placed at the periphery of the target. If the target is large enough for the volume covered by the implant, additional stylet(s) are placed at the center region approximately 10 mm apart from neighboring stylets. Next, hollow polyethylene afterloading catheters (Flex Implant Tubes (6F, 30 cm); Nucletron) are introduced through the stylet in the craniocaudal direction from the dorsum of the tongue to the submandibular region. After removal of the stylets, these catheters are secured with buttons at the submandibular skin surface (Fig. 1). Patients received broad-spectrum antibiotics and steroids during and after the implant procedure.
3. Treatment planning, dose-prescription and treatment deliveryAll patients underwent computed tomography (CT) simulation for the 3D brachytherapy plan. The target definition and dose calculation were performed using the brachytherapy planning system Plato (until February 2010) or Oncentra (February 2010 to present) (Nucletron). The planning target volume (PTV) is defined as the circumferential area connecting the peripheral catheters encompassing the target plus 5-mm margins and was limited to the tumor bed in the craniocaudal direction. In addition, the organs-at-risk, particularly the mandible, were delineated. Modification of the PTV was allowed to exclude a critical organ, particularly the mandible. To define the source positions and calculate the dwell times, 3D treatment planning was performed by 3D reconstruction of the target and surrounding structures, such as the mandible, using CT simulation (Fig. 2). The plan was optimized to deliver the prescription dose (the so-called minimum peripheral dose) to cover at least 95% of the PTV, whereas the dose to the mandible was kept as low as possible to minimize the risks of radio-osteonecrosis. Irradiation was performed by connecting the catheters to our afterloading device (microSelectron HDR; Nucletron). This device uses an 192Ir stepping source, which is moved to different positions, separated by 2.5 mm sequentially in all catheters where it stops for different dwell times. During treatment, wet cotton ball as a spacer and/or customized thin lead plate were placed to reduce radiation exposure to adjacent normal mucosa.
All of the treatments were delivered by HDR IBT using plastic tubes after-loaded with 192Ir with a source strength in the range of 0.05 cGy/m2 < total reference air kerma (TRAK) per fraction < 0.23 cGy/m2. Three different radiation dose schemes were prescribed according to each patient's strategy for controlling the disease (Table 2). In twelve patients (75%), ERT was combined with IBT. The median doses of ERT were 55 Gy/25 fractions for the primary lesion, 50 Gy/25 fractions for elective nodal region and 60 Gy/25 fractions for clinically positive lymph nodes. IBT was administered 2-3 weeks after ERT (median, 18 days). The total dose of IBT was 21 Gy/7 fractions/3 days, 3 Gy/fraction twice a day, delivered at least 6 hours apart. In addition, IBT was administered as the sole treatment to three patients in a total dose of 50 Gy/10 fractions and as a postoperative adjuvant treatment to one patient in a total dose of 35 Gy/7 fractions.
4. Follow-up and statistical analysisEvery patient had dental evaluation before they proceed with radiotherapy. During ERT, patients were followed closely for acute toxicity at weekly intervals. Patients were followed 1 month after brachytherapy, followed by every three months for 2 years, then every 6 months for 3 years and thereafter every year. Patients were evaluated for recurrence both clinically and radiographically using CT, MRI and/or positron emission tomography/CT when indicated. Suspected recurrences were biopsied. Overall survival and local control were calculated from the date of implant placement. The analysis was performed after a median follow-up of 41 months (range, 5 to 145 months). All statistical analyses were performed using STATA ver. 9.0 software (STATA Corp., College Station, TX, USA). The actuarial curves were calculated according to the Kaplan-Meier method.
Results1. Clinical outcomesThe 5-year actuarial overall survival of the entire group was 70%. The mean overall survival time was 105 months. The local control rate (LCR) was achieved in 84% of patients after 3 years, with a plateau seen after 14 months (Figs. 3, 4). All five recurrent cases after initial surgery were successfully salvaged by IBT ± ERT. Two of the eleven patients treated with IBT (± ERT) as the initial primary therapy developed local recurrence approximately 3 and 5 months. One patient had cT2N0M0 tongue cancer, inoperable due to cardiac problem. Total 50 Gy of ERT was given to primary tumor and ipsilateral upper neck followed by total 21 Gy/7 fractions of IBT. As a salvage treatment for the local recurrence, the patient received intra-arterial chemotherapy at an outside hospital; however, he expired 10 months after recurrence. Another patient had cT2N0M0 mobile tongue cancer and received 55 Gy of ERT combined with 21 Gy of IBT. He was lost to follow-up soon after local failure. No tumor or treatment factors, such as primary site, tumor size, stage, treatment modality (brachytherapy +/- ERT), and tumor status (primary vs. recurrent) were significantly correlated with the risks of local recurrence. For instances, the 5-year LCR based on tumor size <3 cm vs. ≥3 cm were 86% vs. 80%, respectively; which were not statistically significant (p = 0.957). Similarly, the treatment modality, IBT alone vs. IBT combined with EBRT did not influence the 5-year LCR (100% vs. 76%, respectively, p = 0.350) (Table 3).
Regional failures were observed in two cases, one with cT4N0M0 tongue cancer and the other with pT2N0M0 tongue cancer. Both of these patients had not received elective neck irradiation because of poor general condition with dementia and previous neck dissection, respectively. They were treated with neck dissection and postoperative neck irradiation as salvage treatments.
2. ComplicationsNone of the patients stopped treatment during the course of brachytherapy. Acute mucositis grade II (Common Terminology Criteria for Adverse Events v4.0) was seen in 5 of 16 patients during treatment. The acute complications associated with IBT (e.g., mucositis, taste and sensory disorders) were not severe enough to interfere with oral intake, and no patient required tube feeding. Mandibular osteoradionecrosis (ORN) were observed in three patients as a late complication (Table 4). One patient with tongue cancer, T4 with mandible involvement, who were treated with IBT alone of 50 Gy/10 fractions developed ORN 24 months after treatment. With the shrinkage of the tumor, mandible was exposed and progressed to bony necrosis and the patient was lost to follow-up. Another patient with unresectable, large (>7 cm) recurrent tumor at the floor of mouth after partial glossectomy, developed ORN about 6 years after treatment of 55 Gy/25 fractions and IBT, ORN was triggered by dental extraction, which is well known as a risk factor for the initiation of ORN. She required partial mandibulectomy with fibula bone graft and is currently alive and well. The other patient with T2 lateral tongue cancer developed ORN 14 months after ERB+IBT. In his case, there was wide superficial lesions adjacent gingiva and floor of mouth. To cover this lesion, almost full dose of radiation was delivered to the gingiva and the floor of mouth; which resulted in mucosal necrosis 5 months after treatment. Mucosal necrosis gradually progressed to ORN. He was treated with sequestrectomy and he is alive and well.
Discussion and ConclusionPreserving the complex functions of the OC, such as degluti-tion, phonation and airway protection, has been a difficult challenge when treating carcinoma in this anatomical region. The treatment modalities available include surgery, ERT, IBT, and various combinations of the three; however, considerable uncertainty remains on choice of treatment. Despite reconstruction, resections may leave considerable functional deficits. In the case of radiotherapy, delivering an adequate dose to the tumor with minimization to surrounding tissues has been a persistent major issue. Brachytherapy is well known for its special characteristics, providing a highly localized dose of radiation, with a rapid fall-off and short overall treatment time [2]. Owing to its properties IBT is the most powerful radiotherapy tool for controlling local disease, even though implantation has a moderate invasiveness.
Several studies have emphasized the superiority of IBT in local control compared with ERT alone. In the early 1980s, Mendenhall et al. [7] published a retrospective analysis of 147 patients with squamous cell carcinoma of the oral tongue and floor of the mouth who were treated with radical radiation therapy. There was an increase in the LCR of oral tongue cancer with treatment by radium alone or radium plus <3,000 rad of external beam radiation compared with radium plus ≥3,000 rad of external beam radiation (p = 0.02). Wendt et al. [8] also reported the treatment results of definitive radiotherapy administered to 103 patients with early-stage oral tongue cancer, which showed that a high proportion of radiotherapy delivered with interstitial brachytherapy is necessary to secure optimum local control of early primary tongue cancer (2-year LCR of 92% with brachytherapy vs. 65% without brachytherapy; p = 0.01). In Japan, Ichimiya et al. [9] analyzed the medical records of 133 patients with stage I tongue cancer and concluded that the addition of IBT significantly improves local control after definitive radiotherapy (with interstitial implant vs. without interstitial implant; 5-year LCR of 89.9% vs. 68.4%, respectively; p = 0.003). Those data provided the rationale to perform IBT in OC cancer patients.
Despite numerous reports demonstrating the effectiveness of IBT, this method has been used less commonly in recent years probably because concurrent chemoradiation has been proven effective in controlling localized tumors. In addition, the advent of new technology in radiotherapy, such as IMRT, results in improved local control and quality of life by sparing the adjacent normal organs [10]. Nevertheless, some results still suggest a unique role for IBT for OC cancers even in this era of IMRT. Recent data have shown that IMRT (with or without concurrent chemotherapy) resulted in an LCR of approximately 60% in OC cancer when including advanced-stage disease cases [11,12]. Sher et al. [11] published a retrospective study of 42 patients treated with adjuvant (n = 30) or definitive (n = 12) IMRT for OC squamous cell carcinoma. Regarding definitive IMRT (±concurrent chemotherapy), 70 Gy radiation was delivered to the primary diseases, and six local failures occurred, resulting in a 2-year LCR of 64%. Compared with the IMRT data, HDR brachytherapy in our study showed a better outcome, with a 3-year LCR of 84%. Sresty et al. [13] compared IBT with IMRT with respect to treatment planning by designing and evaluating both in 15 patients with tongue cancer. They reported that IBT causes equal or superior planning results in regard to the conformity index and dose to critical organs. In addition, Eisbruch et al. [14] reviewed the experiences at two institutions, Michigan (n = 36) and Rotterdam (n = 77), with respect to dysphagia, which is a major late complication of intensive radiotherapy after treating head and neck cancer. They reported that brachytherapy was the only significant factor in their multivariate analysis that reduced dysphagia associated with chemoradiotherapy of head and neck cancer, compared with IMRT. Those results support our notion that brachytherapy still plays a potential role in oral cavity cancers even in this era of IMRT with concurrent chemotherapy.
For patients with a low risk of lymphatic metastasis (T1 or T2, early stage tumors), IBT can be used as a sole treatment; however, for patients with more advanced disease, IBT is usually combined with ERT to treat regional lymphatics. In the latter cases, IBT is commonly administered as a boost [15,16,17]. Furthermore, it is possible to use IBT as salvage treatment for recurrent cases after previous ERT [18]. In the current study, IBT alone was used as salvage treatment for small recurrent lesion (<3 cm) or as a primary treatment for the patient with other comorbidities. However, ERT should be combined with IBT to treat regional lymphatics in cases with risks of occult metastases. According to the literature, the 5-year LCR by IBT is 79%-93% for stage T1 tongue cancer, and 70%-83% for stage T2 [15,19,20,21]. When it comes to the types of IBT, HDR brachytherapy has been challenging LDR brachytherapy with several advantages, such as easier dose optimization and the low risk of radiation exposure to medical personnel. Inoue et al. [6] performed a phase III study that compared LDR IBT (n = 26) with HDR IBT (n = 25) for the treatment of early mobile tongue cancer. The 5-year LCR of the LDR and HDR groups were 84% and 87%, respectively, and they concluded that HDR IBT provides an alternative treatment for LDR IBT. Regarding late toxicity, mandibular bone necrosis induced by LDR IBT has been reported in 7.5%-31% of patients [21,22], and it is still unclear whether HDR IBT is related with more late complications than LDR IBT; some reports have shown an association between HDR IBT and a higher incidence of severe complications (22%) compared with LDR IBT (10%) [17], on the other hand, Leung et al. [23] reported a very low incidence of severe toxicity (5%) after HDR IBT. The current study showed a comparable LCR of 84% after 3 years with a severe late toxicity rate of 3/16 (18%) which was similar to or less than previously reported studies (Table 5). As shown in the results, most of ORN cases have received high doses to the mandible or nearby soft tissue. Therefore, it is important to minimize the dose to these tissues, whenever possible. Although we have been diligent to minimize the dose to the mandible by using a spacer or application of customized thin lead plate, etc., it is sometimes difficult to avoid late complication if the tumor is located close to this structure. Therefore, we should be more cautious about dental care before and after IBT to prevent late complications.
The present study summarizes our experience with HDR IBT of OC cancer. Although this was a retrospective series using a small number of patients, our study showed that HDR IBT demonstrated a favorable local control probability with an acceptable toxicity in diverse treatment settings. In summary, although larger studies are warranted to refine the optimal dose/fractionation and techniques, HDR IBT could be a favorable option for patients with OC cancer as primary or salvage treatment.
References1. Lau HY, Hay JH, Flores AD, Threlfall WJ. Seven fractions of twice daily high dose-rate brachytherapy for node-negative carcinoma of the mobile tongue results in loss of therapeutic ratio. Radiother Oncol 1996;39:15–18, PMID: 8735489.
2. Mazeron JJ, Ardiet JM, Haie-Meder C, et al. GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas. Radiother Oncol 2009;91:150–156, PMID: 19329209.
3. Rudoltz MS, Perkins RS, Luthmann RW, et al. High-dose-rate brachytherapy for primary carcinomas of the oral cavity and oropharynx. Laryngoscope 1999;109:1967–1973, PMID: 10591356.
4. Erickson BA, Demanes DJ, Ibbott GS, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:641–649, PMID: 21106308.
5. Paine CH, Ash DV. Interstitial brachytherapy: past-present-future. Int J Radiat Oncol Biol Phys 1991;21:1479–1483, PMID: 1938556.
6. Inoue T, Inoue T, Yoshida K, et al. Phase III trial of high- vs. low-dose-rate interstitial radiotherapy for early mobile tongue cancer. Int J Radiat Oncol Biol Phys 2001;51:171–175, PMID: 11516867.
7. Mendenhall WM, Van Cise WS, Bova FJ, Million RR. Analysis of time-dose factors in squamous cell carcinoma of the oral tongue and floor of mouth treated with radiation therapy alone. Int J Radiat Oncol Biol Phys 1981;7:1005–1011, PMID: 7298397.
8. Wendt CD, Peters LJ, Delclos L, et al. Primary radiotherapy in the treatment of stage I and II oral tongue cancers: importance of the proportion of therapy delivered with interstitial therapy. Int J Radiat Oncol Biol Phys 1990;18:1287–1292, PMID: 2370178.
9. Ichimiya Y, Fuwa N, Kamata M, et al. Treatment results of stage I oral tongue cancer with definitive radiotherapy. Oral Oncol 2005;41:520–525, PMID: 15878758.
10. Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–136, PMID: 21236730.
11. Sher DJ, Thotakura V, Balboni TA, et al. Treatment of oral cavity squamous cell carcinoma with adjuvant or definitive intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:e215–e222, PMID: 21531515.
12. Daly ME, Le QT, Kozak MM, et al. Intensity-modulated radiotherapy for oral cavity squamous cell carcinoma: patterns of failure and predictors of local control. Int J Radiat Oncol Biol Phys 2011;80:1412–1422, PMID: 20675073.
13. Sresty NV, Ramanjappa T, Raju AK, Muralidhar KR, Sudarshan G. Acquisition of equal or better planning results with interstitial brachytherapy when compared with intensity-modulated radio therapy in tongue cancers. Brachytherapy 2010;9:235–238, PMID: 20116345.
14. Eisbruch A, Levendag PC, Feng FY, et al. Can IMRT or brachy-therapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys 2007;69(2 Suppl):S40–S42, PMID: 17848291.
15. Fujita M, Hirokawa Y, Kashiwado K, et al. Interstitial brachy-therapy for stage I and II squamous cell carcinoma of the oral tongue: factors influencing local control and soft tissue complications. Int J Radiat Oncol Biol Phys 1999;44:767–775, PMID: 10386633.
16. Matsuura K, Hirokawa Y, Fujita M, Akagi Y, Ito K. Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: maximum tumor thickness is prognostic of nodal metastasis. Int J Radiat Oncol Biol Phys 1998;40:535–539, PMID: 9486601.
17. Umeda M, Komatsubara H, Nishimatsu N, Yokoo S, Shibuya Y, Komori T. High-dose rate interstitial brachytherapy for stage I-II tongue cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:667–670, PMID: 11077395.
18. Hepel JT, Syed AM, Puthawala A, Sharma A, Frankel P. Salvage high-dose-rate (HDR) brachytherapy for recurrent head-andneck cancer. Int J Radiat Oncol Biol Phys 2005;62:1444–1450, PMID: 16029806.
19. Shibuya H, Hoshina M, Takeda M, Matsumoto S, Suzuki S, Okada N. Brachytherapy for stage I & II oral tongue cancer: an analysis of past cases focusing on control and complications. Int J Radiat Oncol Biol Phys 1993;26:51–58, PMID: 8482630.
20. Bourgier C, Coche-Dequeant B, Fournier C, et al. Exclusive low-dose-rate brachytherapy in 279 patients with T2N0 mobile tongue carcinoma. Int J Radiat Oncol Biol Phys 2005;63:434–440, PMID: 16168836.
21. Pernot M, Hoffstetter S, Peiffert D, et al. Role of interstitial brachytherapy in oral and oropharyngeal carcinoma: reflection of a series of 1344 patients treated at the time of initial presentation. Otolaryngol Head Neck Surg 1996;115:519–526, PMID: 8969757.
22. Notani K, Yamazaki Y, Kitada H, et al. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck 2003;25:181–186, PMID: 12599284.
23. Leung TW, Wong VY, Kwan KH, et al. High dose rate brachytherapy for early stage oral tongue cancer. Head Neck 2002;24:274–281, PMID: 11891960.
24. Kakimoto N, Inoue T, Inoue T, et al. Results of low- and high-dose-rate interstitial brachytherapy for T3 mobile tongue cancer. Radiother Oncol 2003;68:123–128, PMID: 12972306.
Fig. 1Interstitial catheter implantation procedure in a tongue cancer patient. (A) Stainless steel stylets are inserted through the ipsilateral submental region. (B) Afterloading catheters are introduced through the stylet. (C, D) Catheters are secured with buttons. 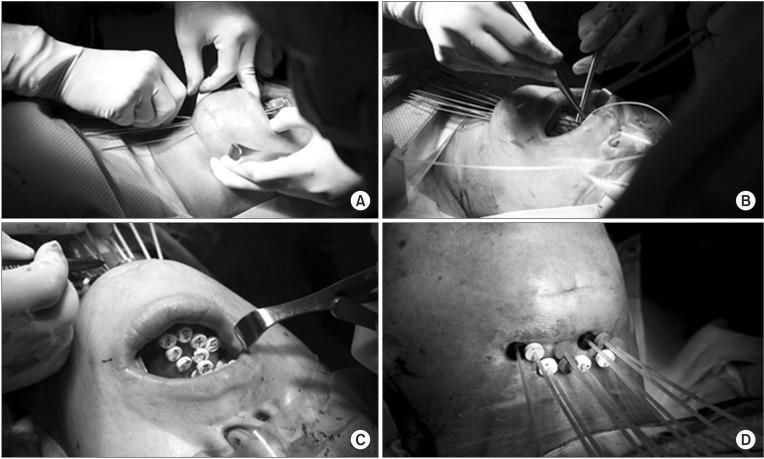
Fig. 2Treatment planning. (A, B) Three-dimensional conformal treatment planning with isodose lines (color codes are shown on the right side). (C) Reconstruction of the target volume, whole implants and mandible. (D) Dose-volume histogram. PTV, planning target volume. 
Fig. 3(A-C) Successful salvage of recurrent tongue cancer in the submental area: (A) pre-treatment status on magnetic resonance imaging; (B) 3 months after treatment, shrinkage of the recurrent tumor (arrow); (C) Afterloading catheter in the submental lesion. (D-F) Complete remission of buccal mucosal cancer after interstitial implantation: (D) pre-treatment status on positron emission tomography; (E) 3 months after treatment, the loss of hypermetabolic lesions in the left buccal mucosa (arrow); (F) Afterloading catheter in the left buccal lesion. 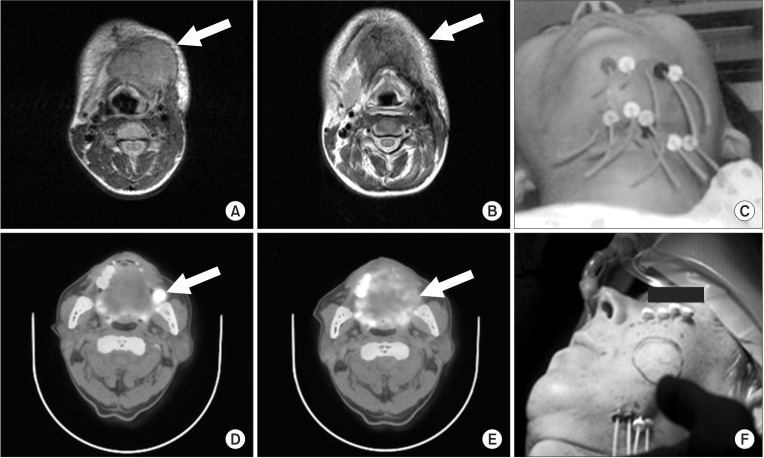
|
|
||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |