 |
 |
AbstractPurposeColorectal cancer is becoming an increasing concern in the middle-aged population of Iran. This study aimed to compare the preliminary results of short-course and long-course neoadjuvant chemoradiotherapy treatment for rectal cancer patients.
Materials and MethodsIn this clinical trial we recruited patients with rectal adenocarcinoma located from 5 cm to 15 cm above the anal verge. Patients in group I (short-course) received three-dimensional conformational radiotherapy with a dose of 25 Gy/5 fractions in 1 week plus concurrent XELOX regimen (capecitabine 625 mg/m2 from day 1ŌĆō5 twice daily and oxaliplatin 50 mg/m2 on day 1 once daily). Patients in group II (long-course) received a total dose of 50ŌĆō50.4 Gy/25ŌĆō28 fractions for 5 to 5.5 weeks plus capecitabine 825 mg/m2 twice daily. Both groups underwent consolidation chemotherapy followed by delayed surgery at least 8 weeks after radiotherapy completion. The pathological response was assessed with tumor regression grade.
ResultsIn this preliminary report on complications and pathological response, 66 patients were randomized into two study groups. Mean duration of radiotherapy in groups I and II was 5 ┬▒ 1 days (range, 5 to 8 days) and 38 ┬▒ 6 days (range, 30 to 58 days). The median follow-up was 18 months. Pathological complete response was achieved in 32.3% and 23.1% of patients in the short-course and long-course groups, respectively (p = 0.558). Overall, acute grade 3 or higher treatment-related toxicities occurred in 24.2% and 22.2% of patients in group I and II, respectively (p = 0.551). No acute grade 4 or 5 adverse events were observed in either group except one grade 4 hematologic toxicity that was seen in group II. Within one month of surgery, no significant difference was seen regarding grade Ōēź3 postoperative complications (p = 0.333).
ConclusionFor patients with rectal cancer located at least 5 cm above the anal verge, short-course radiotherapy with concurrent and consolidation chemotherapy and delayed surgery is not different in terms of acute toxicity, postoperative morbidity, complete resection, and pathological response compared to long-course chemoradiotherapy.
IntroductionColorectal cancer is the third most common cancer in males and the fourth most common among females in Iran [1]. The incidence of rectal cancer in our country is relatively lower than that of Western countries; however, it has been increasing rapidly in recent years. According to a study by Malekzadeh et al. [2], the incidence of colorectal cancer has increased by 80% in the last 30 years in Iran. Interestingly, up to the age of 45 years, the incidence of this cancer in the Iranian population does not significantly differ compared with the US population. Therefore, colorectal cancer is becoming a serious problem for the young Iranian population and the workforce [3].
Currently, the routine treatment plan for locally advanced rectal cancers comprises of preoperative radiation with or without chemotherapy followed by surgery. To date, two types of neoadjuvant therapy have been introduced for rectal cancer. The first method, known as long-course chemoradiotherapy (LCRT) or conventional chemoradiotherapy, includes 45ŌĆō54 Gy in 25ŌĆō28 fractions along with concomitant chemotherapyŌĆömainly 5-fluorouracil (5FU) or its derivativesŌĆöfollowed by delayed surgery 6ŌĆō8 weeks later. This method is mostly applied in the United States and several European countries [4]. Another method is the Northern-European method (especially in Scandinavia and Poland), known as short-course radiotherapy (SCRT), which consists of 25 Gy in 5 fractions without concomitant chemotherapy followed by immediate surgery within 1 week after radiotherapy completion [5].
One of the disadvantages of conventional LCRT with concomitant chemotherapy is the prolongation of the treatment course and the interval between diagnosis and surgery. Moreover, LCRT is costly. Likewise, in our non-private center, patients experience long waiting times for LCRT, whereas the time spent for SCRT is one-fifth of that in LCRT.
In most studies, SCRT without chemotherapy has shown lower pathological response rates compared with conventional LCRT [6], and the addition of chemotherapy to SCRT has always been associated with a concern about increased complications [7]. In addition, no theoretical consensus currently exists on a SCRT regimen that will yield the highest response rate [8,9].
According to our previous studies on short-course and long-course treatment in rectal cancer patients, and the promising results achieved with SCRT, we aimed to compare these two methods in terms of safety profile, pathologic response, and survival in a randomized controlled trial [10,11]. Here, we report the preliminary results of this study by 50% of the expected accrual, including treatment complications and pathological complete response (pCR). In the future, we will report late toxicities and survival rates.
Materials and MethodsThis study was a randomized controlled clinical trial conducted at the radiation oncology ward of Cancer Institute of Iran. Patient recruitment began in April 2016. Patients with a confirmed histological diagnosis of rectal adenocarcinoma located within 5 to 15 cm from the anal verge, and cT3-4 stage or node positive statusŌĆöbased on magnetic resonance imaging (MRI) or endoscopic ultrasound (EUS)ŌĆöwere enrolled in the study. We excluded patients with distant metastasis, an Eastern Cooperative Oncology Group performance score >1, non-operable status or intolerance to chemotherapy, a history of current or past second malignancy, recurrence after previous surgery, and cases of familial adenomatous polyposis (FAP).
The pre-treatment evaluation consisted of imaging modalities such as pelvic MRI, EUS, and thoracoabdominal computed tomography (CT) scan and lab tests including complete blood count, liver and renal function tests, and serum carcinoembryonic antigen (CEA). After completion of staging workup examinations, patients who met the eligibility criteria were given informed consents. Patients who were willing to take part in the study were then randomly assigned either to the short-course or long-course treatment group. Patients of each group were matched in terms of stage of cancer. Randomization was based on permuted block method. Due to the nature of the study intervention, blinding of participants to the assignment group was not possible. In order to minimize patient loss and withdrawal, the investigators followed participants by telephone. After initiation of the study, physical examination and lab tests were performed weekly to assess post-treatment complications. The treatment regimen of group I (short-course) consisted of three-dimensional conformational radiotherapy (3D-CRT) with a total dose of 25 Gy in 5 fractions in 1 week plus concurrent XELOX (capecitabine 625 mg/m2 twice daily from day 1 to 5 and oxaliplatin 50 mg/m2 intravenous injection day 1 only). As for group II (long-course), patients underwent 3D-CRT with a total dose of 50ŌĆō50.4 Gy in 25ŌĆō28 fractions during 5 to 5.5 weeks plus concurrent capecitabine 825 mg/m2 twice daily. Capecitabine tablets were provided by the Actero Middle East Company (a.k.a. Actero Pharma in Tehran, Iran) for all participants. Patients of both groups underwent delayed surgery 8 weeks after the completion of radiotherapy which was performed in either the surgical oncology ward or the colorectal surgery ward. They also received pre-operation chemotherapy with XELOX 3 to 4 weeks after radiotherapy completion.
1. Outcome assessmentThe primary outcome of interest was acute toxicity during chemoradiotherapy up to 1 month of its completion based on the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. In the present report, the secondary outcomes were pCR and down-staging, complete resection, and post-operative morbidity. Pathological response was defined by tumor regression grade (TRG). We used the modified Ryan system that had been endorsed by the American Joint Committee on Cancer [12]. Overall down-staging was defined as conversion of clinical stage to ypT0-2N0, which is considered as non-locally advanced disease. Tumor down-staging was defined as the conversion of primary tumor to ypT0-2. Nodal down-staging was characterized only among those with cN1-2 if ypN was less advanced than cN. Post-operative morbidity was characterized as any toxicity attributable to surgery up to 1 month after the procedure.
2. Treatment planningContouring of clinical target volume (CTV) was based on the Radiation Therapy Oncology Group consensus [13]. Delineation of lymph node basins at risk was based on the international guideline by Valentini et al. [14]. Planning target volume (PTV) was generated by planning software and with adding an 8-mm margin in all dimensions. Before initiation of treatment, the definite treatment plan was approved by the patientsŌĆÖ physician regarding 95% dose coverage of PTV, dose and location of Dmax, and dose to organs-at-risk.
3. Statistical analysisSample size was calculated according to a previous study performed at this center and another study in which the reported incidence of grade Ōēź2 toxicity was 50% and 75% in SCRT and LCRT, respectively [10,15]. The power was 80% and type I error (╬▒) was 0.05. Taking into account a 10% loss, the required sample size was calculated as 120 patients (60 in each group). Since this was a preliminary analysis, we included 50% of the total expected accrual size. We used the following formula to calculate sample size [16]:
n = (Z╬▒/2 + Z╬▓)2 ├Ś (p1(1ŌĆōp1) + p2(1ŌĆōp2)) / (p1ŌĆōp2)2
For evaluating pCR and surgical and chemoradiotherapy complications, the chi-square test and multivariate logistic regression were used. A p-value of less than 0.05 was considered as statistically significant.
4. Ethical considerationsInformed consent was obtained from all participants prior to enrollment in the study. This study was approved by the Ethics Committee of Tehran University of Medical Sciences and the Iranian Registry of Clinical Trials (Ethics Code: IR.TUMS.VCR.REC.1396.3475, IRCTID: IRCT2017110424266N3).
ResultsInitially, 123 patients were recruited; however, after consideration of inclusion and exclusion criteria, only 66 patients were allocated to receive either SCRT (group I) or LCRT (group II). No patient was lost to follow-up or discontinued intervention (Fig. 1).
1. Baseline characteristicsThe baseline characteristics of patients in both groups are demonstrated in Table 1. As shown, there was no significant difference between the two groups in respect to the studied variables. The median age of patients in the short-course and long-course treatment groups was 56 ┬▒ 10.3 and 53 ┬▒ 12.9 years old, respectively. In both groups, the majority of patients had a histologic grade 1 tumor.
2. Acute treatment toxicityThe mean duration of radiotherapy course in the SCRT and LCRT groups was 5 ┬▒ 1 and 38 ┬▒ 6 days, respectively. Grade 2 and higher acute adverse events (AEs) were observed in 75.8% and 61.5% of patients in group I and II, respectively (p = 0.19). The percentage for grade 3 and higher AEs in the concurrent chemoradiotherapy period was 15.2% and 14.8%, respectively (p = 0.63). No grade 4 or 5 radiotherapy-induced adverse event was observed except one grade 4 hematologic toxicity in the LCRT group.
Grade 3 and higher AEs related to preoperative chemotherapy (consolidation) were observed in 12.1% and 11.5% of patients in group I and II, respectively (p = 0.64). Preoperative chemotherapy tolerance (receiving full planned dose) in group I and II was 87.9% and 81.8%, respectively (p = 0.4). Collectively, grade 3 or higher acute treatment toxicities acute treatment toxicities, including chemoradiotherapy- and consolidation chemotherapy-attributable AEs, were seen in 24.2% and 22.2% of patients in group I and II, respectively (p = 0.55).
The frequency of the most severe acute toxicities is demonstrated in each treatment group (Table 2).
3. Surgical outcomesFifty-five percent of the surgical specimens were re-examined by a skilled pathologist. TRG was altered in 6/33 (18.2%) cases; of note, however, only one change from grade 1 to grade 0 was observed, and the majority of changes were between grades 2 and 3. A pCR (final TRG = 0) in the SCRT and LCRT groups was achieved in 32.2% and 23.1% of cases, respectively (p = 0.56); whereas a pathological complete or near complete response (final TRG = 0ŌĆō1) was seen in 41.9% and 42.3% of cases, respectively (p = 0.99). One patient had ypT0N1 that was considered TRG = 0, however, his/her surgical stage was classified as stage 2. Tumor down-staging (ypT0-2 ypN0, based on the definition) occurred in 54.8% and 53.8% of the cases in group I and II, respectively (p = 0.58). Detailed data is shown in Table 3.
4. Post-operative morbidityThe frequency of grade 3 or higher post-operative morbidities (within 1 month after surgery) in the SCRT and LCRT groups was 19.4% and 11%, respectively (p = 0.33). No post-operative mortality was observed. The type of post-operative morbidities in each group is shown in Table 4.
5. Late treatment toxicityThe frequency of grade 2 or higher late treatment toxicities (at least 6 months after radiotherapy) in the SCRT and LCRT groups were 38.7% and 38.4%, respectively (p = 0.56); while grade 3 and higher toxicities were seen in 6.5% and 11.5% of the patients in group I and II, respectively (p = 0.16) (Table 5). The frequency of late treatment toxicities varied based on the ward in which surgeries were performed (Table 6); in the colorectal surgery ward, toxicities were less frequently observed in patients who received the short-course treatment compared with patients who received long-course treatment (p = 0.02).
Discussion and ConclusionWe evaluated the preliminary outcomes of SCRT with concomitant XELOX regimen in comparison to LCRT with concomitant capecitabine. Both study groups received consolidation chemotherapy with XELOX at the resting interval before surgery. The reason for administering XELOX in the concurrent regimen of patients undergoing SCRT was the promising results we achieved in the previous single-arm study conducted at our institution [10]. Also, another study published a few years ago showed that the addition of oxaliplatin leads to more robust down-staging compared with capecitabine alone [17]. Considering these results, we aimed to investigate the efficacy and safety of XELOX regimen for concurrent chemotherapy in SCRT as a potential alternative option compared with the standard treatment, which is LCRT with fluorouracil or capecitabine. As for the consolidation regimen, we used the adjuvant therapy for locally advanced rectal cancer, which is primarily XELOX, since oxaliplatin has been reported to enhance disease-free survival compared with capecitabine alone or fluorouracil plus leucovorin alone [18,19].
Some patients experienced a relatively long interval from radiation completion to surgery. These outliers were either due to temporary loss to follow-up, long waiting lists of the surgical wards, or occasional lack of coordination between the departments. Although none of the patients experienced re-growth of the tumor at the time of surgery, this issue should be taken into consideration since interval prolongation might affect pathological response.
In this study, there was no significant difference in respect to acute treatment toxicities and post-operative morbidities between the short-course and long-course treatment groups. In LCRT, the peak of radiotherapy adverse events, including proctitis, enteritis, diarrhea, and mucositis is within the third to fifth week of treatment and the severity of the acute toxicities will gradually decrease 3 to 4 weeks after the completion of therapy. In this group, the patient receives chemotherapy simultaneously along with radiotherapy at the time when toxicities are at their peak, which can affect the severity of toxicities [20]. It is assumed that more acute toxicities are faced in the short-course treatment which uses the hypofractionated regimen, as it is a kind of accelerated regimen. According to a previous Polish study, in SCRT, acute toxicities peak at the 11th to 14th day after radiotherapy. At this time, the patient is resting after a 5-day treatment course, so he/she is not exposed to chemotherapy or radiotherapy; on the other hand, delaying the surgery for at least 8 weeks after irradiation will reduce the severity of toxicities [21]. In SCRT, malignancy symptoms (obstruction, bleeding, rectal discomfort) resolve sooner due to larger fractions and thus, influence the decline in the feeling of discomfort from acute toxicities. The lack of statistically significant difference in acute toxicities between the two groups can be explained by the mentioned reasons.
Another interesting finding of this study was that despite a smaller equieffective dose of radiotherapy (EQD2 = 31.25 Gy vs. 50 Gy) and also a smaller total chemotherapy dose (5 days vs. 25 days of capecitabine delivery) in the SCRT group, short-term oncologic outcomes were the same in both groups. More interestingly, in the SCRT group, the pCR was higher than that in most of the previous similar studies [22-27]. This finding can be rationalized by the fact that the large fractions used in a hypofractionated radiotherapy regimen can induce an immune response that will eventually increase the biologic effects of concomitant and consolidation chemotherapy. This immune response results from the release of a great number of antigens due to the breakdown of tumoral cells, and the presentation of these antigens to T cells [28,29]. Nevertheless, this hypothesis needs further assessment.
Comparison of our study with other similarly designed studies in which chemotherapy is applied along with SCRT concluded that we had reached more favorable outcomes (Table 7). So, the question is why short-course chemoradiation has produced such a good result. There are several reasons that could explain the more favorable results in this study; firstly, the precise delineation of target volumes based on international guidelines [14] and the strict confirmation of treatment plans, considering the sufficient coverage of PTV and also the dose of organs-at-risk; second, administration of capecitabine with radiotherapy instead of bolus 5FU is shown to be associated with fewer toxicities and higher response rates in a study by Haddad et al. [30]; third, prolonging the interval between radiotherapy completion and surgery to more than 8 weeks, as this has been demonstrated by Rega et al. [31] to reduce adverse events and increase response to neoadjuvant therapy. In our study, the interval from end of radiotherapy to surgery was higher compared with the majority of other similar studies. Consistent with our trial, a study by Myerson et al. [26], with a similar interval of 17 weeks, reported pathological responses comparable to our results; moreover, delivering consolidation chemotherapy before surgery which has been shown to be associated with an increase in complete response rates in a study by Habr-Gama et al. [32]; addition of oxaliplatin to capecitabine simultaneously with radiotherapy, which despite controversies, has been proved to increase response rates [17]; and lastly, performing operations in a more specialized ward for colorectal surgery by skilled and experienced colorectal surgeons.
In conclusion, SCRT versus LCRT with consolidation chemotherapy and delayed surgery were not significantly different in regards to acute toxicities, post-operative morbidities, complete resection, and pathological response. However, we should wait longer to be able to make a definitive comment on local recurrence, distant recurrence, survival rates, and late toxicities. Since our patients were only followed for a relatively short period of 18 months, results on these long-term measures will be reported separately in future studies. Prospective studies should focus on using novel radiotherapy techniques for reducing grade 2 acute and late AEs. Moreover, comparison of SCRT with sequential versus simultaneous chemotherapy, investigation of tumor and mesorectum dose-escalation with SCRT to increase pCR rates, and implementation of a watch-and-wait approach after SCRT + chemotherapy is suggested. Conclusively, re-performing the current study with removal of simultaneous oxaliplatin, and based on the substantial pCR, and investigating sphincter preservation in lower rectal tumors (less than 5 cm from the anal verge) is recommended.
AcknowledgmentsWe thank the Actero Middle East Company (a.k.a. Actero Pharma) providing the capecitabine tablets for both groups.
This study was funded by the Vice-chancellor of Research, Tehran University of Medical Sciences (Grant ID: 95-04-207-33683).
Fig.┬Ā1.CONSORT flow diagram of the study. AV, anal verge; PS, performance score; ChT, chemoradiotherapy; FAP, familial adenomatous polyposis; ITT, intention-to-treat. 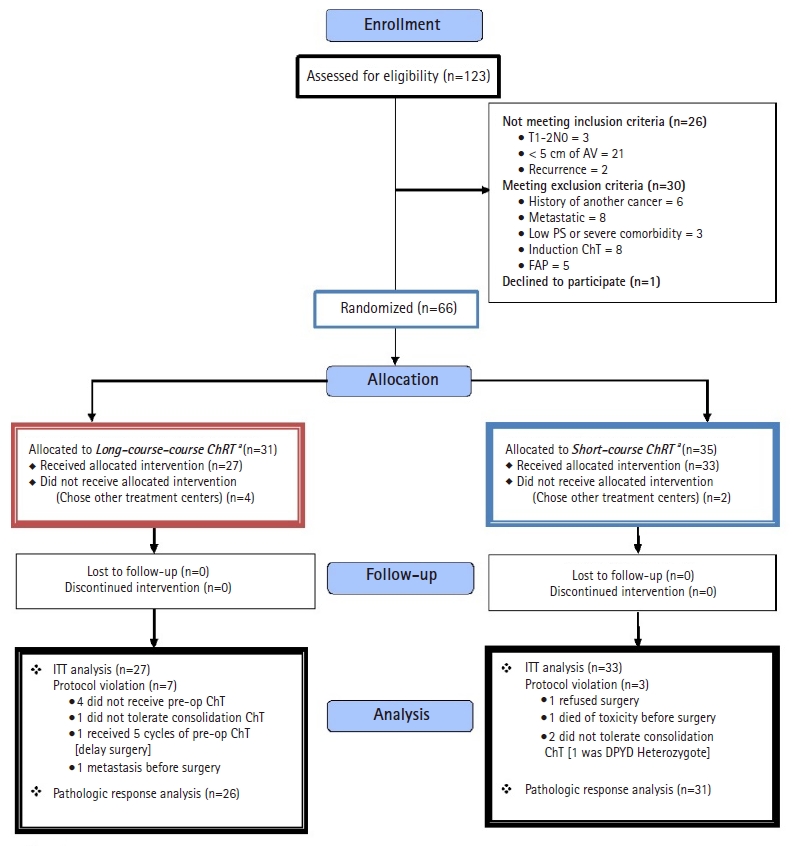
Table┬Ā1.Baseline characteristics of patients in both treatment groups Table┬Ā2.Frequency of the most severe acute toxicities in SCRT (group I) and LCRT (group II) before consolidation chemotherapy Table┬Ā3.Characteristics of surgeries and response to neoadjuvant treatment in the SCRT and LCRT groups Table┬Ā4.Frequency and type of postoperative morbidities in SCRT and LCRT groups
Table┬Ā5.Late treatment toxicities and frequency of the most severe complications in SCRT (group I) and LCRT (group II) Table┬Ā6.Comparison of late treatment toxicities (grade 3 or higher) between colorectal surgery ward and surgical oncology ward
Table┬Ā7.Comparison of previous similarly designed studies with this study
References1. Rafiemanesh H, Pakzad R, Abedi M, et al. Colorectal cancer in Iran: epidemiology and morphology trends. EXCLI J 2016;15:738ŌĆō44.
2. Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular genetics of colorectal cancer in Iran: a review. Arch Iran Med 2009;12:161ŌĆō9.
3. Hessami Arani S, Kerachian MA. Rising rates of colorectal cancer among younger Iranians: is diet to blame? Curr Oncol 2017;24:e131ŌĆōe137.
4. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114ŌĆō23.
5. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644ŌĆō50.
6. Liu SX, Zhou ZR, Chen LX, Yang YJ, Hu ZD, Zhang TS. Short-course versus long-course preoperative radiotherapy plus delayed surgery in the treatment of rectal cancer: a meta-analysis. Asian Pac J Cancer Prev 2015;16:5755ŌĆō62.
7. Minsky BD, Rodel C, Valentini V. Short-course radiation versus long-course chemoradiation for rectal cancer. J Natl Compr Canc Netw 2012;10:1223ŌĆō31.
8. Margalit O, Mamtani R, Lawrence YR, et al. Locally advanced rectal adenocarcinoma: are preoperative short and long course radiotherapy truly equivalent? Mol Clin Oncol 2019;10:555ŌĆō9.
9. McCarthy K, Pearson K, Fulton R, Hewitt J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 2012;12:CD008368.
10. Aghili M, Sotoudeh S, Ghalehtaki R, et al. Preoperative short course radiotherapy with concurrent and consolidation chemotherapies followed by delayed surgery in locally advanced rectal cancer: preliminary results. Radiat Oncol J 2018;36:17ŌĆō24.
11. Aghili M, Farhan F, Babaei M, et al. Outcomes of neoadjuvant radiochemotherapy of locally advanced rectal adenocarcinoma: results of 8 year experience in Iran Cancer Institute. Int J Cancer Manag 2017;10:e12339.
12. Kim SH, Chang HJ, Kim DY, et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat 2016;48:998ŌĆō1009.
13. Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824ŌĆō30.
14. Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol 2016;120:195ŌĆō201.
15. Parekh A, Truong MT, Pashtan I, et al. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res 2013;6:137ŌĆō43.
16. Wang H, Chow SC. Sample size calculation for comparing proportions. In : Kotz S, Balakrishnan N, Read CB, Vidakovic B, editors. Encyclopedia of statistical sciences. Hoboken, NJ: John Wiley & Sons; 2004.
17. Haddad P, Miraie M, Farhan F, et al. Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: a randomized clinical trial. Asia Pac J Clin Oncol 2017;13:416ŌĆō22.
18. Rodel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979ŌĆō89.
19. Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245ŌĆō53.
20. Zelefsky MJ, Daly ME, Valicenti RK. Low-risk prostate cancer. In : Brady LW, Perez CA, Wazer DE, editors. Perez & Brady's principles and practice of radiation oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013, p. 1280ŌĆō1311.
21. Bujko K, Partycki M, Pietrzak L. Neoadjuvant radiotherapy (5 ├Ś 5 Gy): immediate versus delayed surgery. Recent Results Cancer Res 2014;203:171ŌĆō87.
22. Yeo SG, Oh JH, Kim DY, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01). Int J Radiat Oncol Biol Phys 2013;86:34ŌĆō9.
23. Beppu N, Matsubara N, Kakuno A, et al. Feasibility of modified short-course radiotherapy combined with a chemoradiosensitizer for T3 rectal cancer. Dis Colon Rectum 2015;58:479ŌĆō87.
24. Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 ├Ś 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016;27:834ŌĆō42.
25. Chung MJ, Kim DW, Chung WK, et al. Preoperative short- vs. long-course chemoradiotherapy with delayed surgery for locally advanced rectal cancer. Oncotarget 2016;8:60479ŌĆō86.
26. Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys 2014;88:829ŌĆō36.
27. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827ŌĆō33.
28. Napolitano M, D'Alterio C, Cardone E, et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 2015;6:8261ŌĆō70.
29. Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett 2015;368:185ŌĆō90.
30. Haddad P, Farsiani AR, Dadpour M, Yari H, Safaei AM, Miraei M. Side effects and disease-free survival with capecitabine compared to 5FU for concurrent radiochemotherapy of rectal cancer: a 5-year review. Basic Clin Cancer Res 2014;6:11ŌĆō5.
31. Rega D, Pecori B, Scala D, et al. Evaluation of tumor response after short-course radiotherapy and delayed surgery for rectal cancer. PLoS One 2016;11:e0160732.
32. Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, Sao Juliao GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum 2009;52:1927ŌĆō34.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |