 |
 |
AbstractPurposeThe aim of this study was to evaluate the effectiveness of palliative radiation therapy (RT) for superior vena cava (SVC) syndrome from lung cancer and to compare the 2-week and 1-week schedules.
Materials and MethodsA retrospective study was conducted on lung cancer patients with palliative RT for SVC syndrome. Patients received 30 Gy in 10 fractions (2-week group) or 20 Gy in 5 fractions (1-week group) between July 2012 and June 2022. Treatment outcomes were evaluated at 1 to 2 months after RT. The tumor response and recanalization were evaluated based on the computed tomography (CT).
ResultsOf the 39 patients, 24 received a 2-week course RT and 15 received a 1-week course of RT. The most common SVC-associated symptoms were edema (51.3%) and dyspnea (43.6%). There were no significant differences in performance status, histology, and grade of SVC. Symptom relief in symptomatic patients was comparable (85.7% in the 2-week group vs. 91.6% in the 1-week group; p = 0.581). There were no significant differences between the 2-week and 1-week groups in recanalization rates (62.5% vs. 60.0%; p = 0.876), tumor responses (75% vs. 60.0%; p = 0.876), and 6-month overall survival rates (29.2% vs. 36.4%; p = 0.726). In each of the two groups, one patient was consulted for re-irradiation. The median survival were 3.7 months for the 2-week group and 4.4 months for the 1-week group.
IntroductionSuperior vena cava (SVC) syndrome is a condition caused by the obstruction of blood flow from the head, arms, and upper torso to the heart. Approximately 90% of SVC obstructions are related to thoracic malignancies, which externally compress the SVC with a mass effect or directly invade the SVC [1]. This obstruction leads to increased venous pressure, resulting in symptoms with a wide range of severity, from face and arm swelling to life-threatening symptoms such as central airway obstruction, severe laryngeal edema, and cerebral edema with confusion. Lung cancer is the most common malignancy causing SVC syndrome, accounting for more than 70% of all cases [1,2]. The incidence of SVC obstruction at presentation is 4.2% in patients with lung cancer and is more frequent in small cell lung cancer (SCLC) than in non-small cell lung cancer (NSCLC) [3].
Various treatment options are available for the management of SVC syndrome, including steroids, chemotherapy, intervention, and radiation therapy (RT). Endovascular recanalization is the standard first-line treatment for patients with life-threatening symptoms [4-6]. Intravascular stenting provides rapid relief from symptoms. However, most cases of SVC syndrome are not considered an oncologic emergency and do not require urgent management [7]. In patients without life-threatening symptoms, palliative RT is an effective treatment for symptom relief, particularly in those who are unsuitable for stent insertion or have a poor response to prior chemotherapy. Approximately 70%вҖ“90% of patients with SVC syndrome who receive palliative RT achieve symptom relief [8-10]. However, the optimal dose of palliative RT for SVC syndrome is not well established, and various RT regimens have been considered. The current recommendation by the National Comprehensive Cancer Network suggests a typical palliative RT dose of 30вҖ“45 Gy administered over 2вҖ“3 weeks [11]. However, a shorter course of RT is recommended for patients with short life expectancies. Limited data is available regarding RT regimens for SVC syndrome, particularly in patients with lung cancer. There have been no comparisons of treatment outcomes according to the radiation course, and there have been only a few reports on the treatment outcomes of a typical palliative dose of 30 Gy administered over 2 weeks. Therefore, this study aimed to evaluate the treatment outcomes of palliative RT for SVC syndrome in lung cancer patients and to compare the effectiveness of 1-week and 2-week RT schedules.
Materials and Methods1. PatientsThis retrospective study was approved by the Institutional Review Board of our institution of Kyungpook National University Chilgok Hospital (No. 2020-04-051). The requirement for informed consent was waived because of the retrospective nature of the analyses. We reviewed the medical records of patients with lung cancer who underwent palliative RT for SVC syndrome between July 2012 and June 2022 at our institution. Patients included were treated with either a 2-week course (30 Gy in 10 fractions) or a 1-week course (20 Gy in 5 fractions) of palliative RT. During the study period, 46 patients with SVC syndrome caused by lung cancer were treated with palliative RT. Of these, seven patients were excluded from the analysis for the following reasons: four due to the use of alternative dose-fractionation regimens other than the standard 1-week or 2-week courses, two due to a lack of follow-up evaluation and one due to discontinued RT. No patients died during RT. The remaining 39 patients were enrolled in this study. All patients had a diagnostic chest computed tomography (CT) image at the time they were referred for palliative RT. Among them, 24 received 30 Gy in 10 fractions (2-week group), whereas 15 received 20 Gy in 5 fractions (1-week group). Patient characteristics and treatment details, including radiation dose, fraction size, and treatment response, were obtained from medical records. Lung cancer diagnosis was based on histological confirmation. SVC syndrome diagnosis was based on clinical symptoms or signs, and SVC obstruction observed on CT images. The severity of SVC syndrome was evaluated using the grading system proposed by Yu et al. [12] (Supplementary Table S1).
2. RadiotherapyCT simulation was performed for all patients in the supine position, under free breathing three-dimensional CT. The gross tumor volume (GTV) included the tumor causing the SVC obstruction through encasement or direct invasion. The clinical target volume (CTV) was generated by adding a 5-mm isotropic margin to the GTV. CTV could be tailored to the size and extent of the tumor. If the tumor was too large to be treated in its entirety, the doctor determined the extent of the target volume. The CTV generally included the area from the upper border of the right atrium to the thoracic inlet. The planning target volume was created by expanding the CTV by 5 mm or more. RT planning was performed using the Eclipse RT planning system (Varian Medical Systems, Palo Alto, CA, USA). Three-dimensional conformal RT (3D-CRT) planning was mainly used and based on the physician's preference or the need for organ preservation, intensity-modulated radiotherapy or volumetric-modulated arc therapy techniques were also employed. RT was delivered using 6- or 10-MV photons from a linear accelerator (Varian Medical Systems). The total dose and number of fractions were determined based on the patientвҖҷs condition and the physicianвҖҷs preference. Both RT schedules continuously delivered treatment once daily for 5 days a week.
3. Evaluation and statisticsFollow-up examinations included history taking, physical examination, laboratory tests, CT, magnetic resonance imaging, and positron emission tomography/CT (or when metastasis was suspected), depending on the patientвҖҷs condition, stage, and risk factors. The interval of follow-up was also adjusted depending on the patient and the disease. Recanalization was defined as the restoration of flow in patients with complete obstruction before RT and an increase in the luminal diameter of at least 20% in patients with incomplete obstruction (a visible contrast agent on the SVC on initial CT) before RT. The tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors v1.1 compared to the baseline on a follow-up CT scan. Additionally, the overall tumor response is defined as more than a partial response. Cause of death was evaluated with medical record. The immediate cause defined as the final disease or condition resulting in death. According to the definition provided by Nichols et al. [13] tumor burden was defined as cause of death, resulting from (1) cachexia, (2) no other specific and/or pathophysiologic mechanisms of death, and/or (3) the lung tumor volume being the primary factor leading to fatal respiratory failure.
Differences between the 1-week and 2-week RT courses were compared using Pearson chi-square test for parametric variables. Overall survival (OS) was estimated using the Kaplan-Meier method, and survival duration was calculated from the starting date of RT until the date of death or the last follow-up. Logistic regression was used to evaluate factors influencing symptom relief. Univariate and multivariate analyses were performed using the Cox proportional hazards model to determine the association between the clinical factors and survival outcomes. Backward selection Cox regression analysis was performed using a multivariate model. Statistical analyses were performed using SPSS version 22 (IBM, Armonk, NY, USA) and the R software (R Foundation for Statistical Computing, Vienna, Austria).
ResultsPatient characteristics are shown in Table 1. Of total the 39 patients, 26 (66.7%) had NSCLC and 13 (33.3%) had SCLC. The onset of SVC syndrome in the 2-week group occurred in 70.8% of cases due to disease progression following previous treatment, and the remaining 29.2% occurred at the time of the initial diagnosis of lung cancer. Of the 1-week patients, 53.3% occurred at the time of initial lung cancer diagnosis, and the remaining 46.7% occurred due to disease progression. And in both groups, patients had distant metastasis (70.8% in the 2-week group and 93.3% in the 1-week group; p = 0.090). The most common symptoms of SVC syndrome were edema (51.3%) and dyspnea (43.6%), while 15.4% of the patients were asymptomatic. SVC syndrome grades 0вҖ“2 accounted for the majority of patients in both groups (91.7% in 2-week group and 86.7% in 1-week group; p = 0.617). Steroids and chemotherapy were administered to the majority of patients. In both groups, 3D-CRT was the most commonly used RT planning technique (83.3% in 2-week group and 93.3% in 1-week group).
At 1вҖ“2 months following the completion of RT, among the symptomatic patients (excluding those who were asymptomatic), 87.8% experienced symptom relief (Table 2). Reduction in SVC-associated symptoms was observed in 85.7% of the 2-week group and 91.6% of the 1-week group (p = 0.581) in symptomatic patients. The recanalization rates were 62.5% in the 2-week group and 60.0% in the 1-week group (p = 0.876), and the tumor response rates were 75.0% and 60.0%, respectively (p = 0.323). Consultations for re-irradiation of SVC were observed in one patient from the 2-week group and one patient from the 1-week group. The patient who received the 2-week course of RT was consulted for retreatment 5 months after completing RT. This patient's treatment was interrupted due to hospital-acquired pneumonia after receiving 21 Gy in 7 fractions. The patient who received the 1-week course of RT was consulted 2 months after RT and did not receive re-irradiation determined by the radiation oncologist. Logistic regression analysis indicated that the RT schedule, use of steroids, and concurrent chemotherapy did not have a significant impact on symptom relief (Table 3). At the time of analysis, 35 patients had died. Of these, six patients died outside of the hospital, such as in hospice care, so we could not evaluate the cause of death for them. Of the remaining 29 patients, 28 died of cancer-specific causes, while one died attributed to other specific cause, which was renal failure from underlying chronic kidney disease (Supplementary Table S2). Among the 28 patients who died from cancer-specific reasons, the immediate causes of death were tumor burden in 10 patients, pneumonia in eight patients, and metastatic complications in eight patients. Dyspnea (55.2%) and general weakness (20.7%) were frequently observed. However, no patients were determined to have died as a direct result of SVC syndrome. The mean follow-up duration was 6 months. The median survival was 3.7 months in the 2-week group (95% confidence interval [CI], 2.020вҖ“5.380) and 4.4 months in the 1-week group (95% CI, 2.612вҖ“6.188). Not finding a statistically significant difference in the OS rate based on the RT schedule (29.2% in the 2-week group and 36.4% in the 1-week group; p = 0.726) (Fig. 1). Both univariate and multivariate analyses showed that the treatment schedule was not related to OS (Table 4).
Discussion and ConclusionOur study demonstrated that the 2-week and 1-week course of palliative RT were equally effective for the relief of symptoms of SVC syndrome caused by lung cancer. In symptomatic patients, symptom relief was achieved in 85.7% of the 2-week group and 91.6% of the 1-week group, with no significant difference. And there was no statistically significant difference in recanalization, tumor response, and overall survival between the 2-week course RT and the 1-week course RT.
Historically, palliative RT has been widely advocated for SVC syndrome. However, currently, the SVC stenting has emerged as the first line treatment for SVC obstruction, offering symptom relief in up to 89%, with a stent patency rate of more than 90% [14-16]. This intervention is particularly crucial for patients who require urgent management, with life-threatening symptoms such as larynx swelling, airway obstruction, and cerebral edema [12]. Endovascular stenting can provide more rapid palliation compared to radiation therapy and chemotherapy. However, despite the advantages of the SVC stenting, it is not suitable for all patients. Stenting can be challenging for those with bleeding tendencies due to thrombocytopenia caused by previous treatments or underlying comorbidities. In addition, the lack of necessary equipment or specialists in some medical facilities may further limit its use. For these conditions, palliative RT may still be a good option. Previous studies have demonstrated that RT, using various dose-fractionation schemes, is effective in relieving symptoms of SVC syndrome, with symptom relief achieved in 70%вҖ“90% of patients [8-10]. In our study, a reduction in SVC-associated symptoms was achieved in 87.5% of symptomatic patients. These results were consistent with a previous study. Furthermore, the 1-week course RT showed an equivalent effect on symptom reduction to the 2-week course RT.
Patients with SVC syndrome caused by malignancy are known to have a poor prognosis, with a median life expectancy of approximately 6 months [1]. Our study also showed a poor prognosis, with a median survival of 3.7 months in the 2-week group and 4.4 months in the 1-week group. By the time a patient with SVC syndrome is referred for palliative RT, most patients are already in the advanced stage of the disease, having experienced the failure of prior treatments and/or the presence of distant metastases. Furthermore, we found that tumor response was not associated with OS in both univariate and multivariate analyses. Recanalization of the SVC also had no effect on OS. Most of the patients in our study had grade 0вҖ“2 SVC syndrome and may have already developed collaterals to compensate for SVC occlusion prior to RT [17,18].
The major concern with a 1-week course of palliative RT is that symptom relief could be of shorter duration of palliation effects compared to a longer course, leading to the need for additional interventions. The selection of palliative treatment should encompass not only the reduction of patient complaints but also the evaluation of symptom stability and the duration of palliation effects [19]. In previous palliative RT studies, the durability of the treatment effect between short and long course regimens appears to vary depending on the metastatic site and cancer type. In painful bone metastases, a single fraction of 8 Gy has been shown to be as safe and effective as a longer course of palliative RT (30 Gy in 10 fractions) in terms of pain relief [20]. However, the retreatment rate in the group receiving a single dose of 8 Gy was much higher than that in the 30 Gy in 10 fractions group (28% vs. 2%). Similarly, Olafsdottir et al. [21] found that a short course (20 Gy in 5 fractions) for esophageal cancer had a retreatment rate of 32.0% compared with 18.9% for a long course (30 Gy in 10 fractions). Conversely, Rades et al. [22] found that a 1-week course (20 Gy in 5 fractions) and a 2-week course (30 Gy in 10 fractions) for metastatic epidural spinal cord compression were similarly effective in motor function, ambulatory rate, and local progression-free survival and maintained effects for up to 6 months. In our study, we were unable to directly analyze the sustainability of treatment effect. It was challenging to discern, based on medical records, whether symptoms originated from a recurrence or aggravation of SVC syndrome or from systemic disease progression. In addition, most patients were in end-stage disease. Radiological assessment of tumor progression was also challenging as few patients had serial CT scans due to their short survival time.
Other considerable short-course RT regimens, such as 16вҖ“17 Gy delivered in 2 fractions, have been previously reported effective as palliative thoracic RT. Kramer et al. [23] compared the effectiveness of 16 Gy in 2 fractions and 30 Gy in 10 fractions regimens for palliative RT in patients with NSCLC experiencing hemoptysis, chest pain, dysphagia, and dyspnea. They reported that although 30 Gy in 10 fractions maintained a longer treatment effect and showed better survival, 16 Gy in 2 fractions was equally effective in relieving symptoms over the initial 39 weeks. Sundstrom et al. [24] compared 17 Gy in 2 fractions (daily 8.5 Gy) as a palliative thoracic dose with 42 Gy in 15 fractions (daily 2.8 Gy) and 50 Gy in 25 fractions. They concluded that 17 Gy in 2 fractions is equally effective in symptom palliation and survival in advanced NSCLC patients. Additionally, a study by Senkus-Konefka et al. [25] evaluated the efficacy of two different RT regimens 20 Gy in 5 fractions and 16 Gy in 2 fractions, for patients with inoperable symptomatic NSCLC. Their analysis showed no significant difference in symptom relief between the two RT regimens, indicating similar effectiveness. Although there is limited evidence to support the use of 2-fraction RT for SVC syndrome, its potential as a shorter and more convenient treatment option could make it a viable choice for selected patients. Further research is needed to establish its efficacy and safety.
This study has several limitations. First, as a retrospective study, it may be prone to inherent bias and heterogeneity between the two groups, and the small sample size may have limited the robustness of the findings. However, considering the lack of reports on the optimal palliative dose for lung cancer patients with SVC syndrome, the study findings may prove valuable in guiding clinical treatment decisions. Second, SVC syndrome is typically observed in patients with locally advanced or metastatic disease. These patients represent a heterogeneous group with complicating comorbidities and complex clinical scenarios in which various treatments overlap. Thus, stratifying these patients or distinguishing the sole efficacy of palliative RT is challenging. Similarly, it was not possible to distinguish from the medical record whether symptoms following radiation were due to recurrence or aggravation of SVC syndrome or systemic disease progression. This limitation prevented analysis of the duration of symptom relief. Finally, Treatment-related toxicity is one of major considerations in therapeutic decision-making. However, RT-related toxicity could not be assessed due to the retrospective design of this study. Moreover, distinguishing the cause of their symptom was challenging as they might be multifactorial, arising from RT, other treatments, or the progression of the disease.
In conclusion, a 1-week course of RT for SVC syndrome caused by lung cancer offered equivalent effectiveness in symptom relief, tumor response, and recanalization compared to a 2-week course of RT. There was no significant difference in the consultation for re-irradiation. Given the poor prognosis for lung cancer patients with SVC syndrome, a 1-week course of RT could be a viable option for efficient symptom management.
NotesStatement of Ethics This retrospective study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of our institution of Kyungpook National University Chilgok Hospital (No. 2020-04-051). The informed consent was waived because of the retrospective nature of this study. Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2023.00626.
TableВ S1.Grading systema) for superior vena cava syndrome Fig.В 1.Kaplan-Meier estimates of overall survival for 1-week course and 2-week course palliative radiation therapy for lung cancer with superior vena cava syndrome. 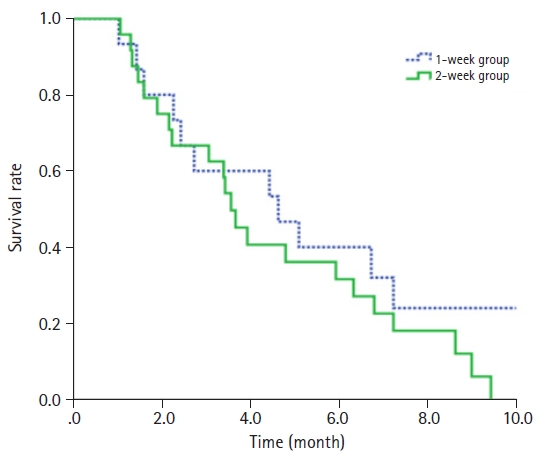
TableВ 1.Patient characteristics TableВ 2.Treatment outcomes TableВ 3.Factors affecting symptom relief TableВ 4.Factors affecting overall survival after RT References1. Wilson LD, Detterbeck FC, Yahalom J. Clinical practice: superior vena cava syndrome with malignant causes. N Engl J Med 2007;356:1862вҖ“9.
2. Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37вҖ“42.
3. Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14:338вҖ“51.
4. Lanciego C, Pangua C, Chacon JI, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol 2009;193:549вҖ“58.
5. Fagedet D, Thony F, Timsit JF, et al. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Intervent Radiol 2013;36:140вҖ“9.
6. Niu S, Xu YS, Cheng L, Cao C. Stent insertion for malignant superior vena cava syndrome: effectiveness and long-term outcome. Radiol Med 2017;122:633вҖ“8.
7. Schraufnagel DE, Hill R, Leech JA, Pare JA. Superior vena caval obstruction: is it a medical emergency? Am J Med 1981;70:1169вҖ“74.
8. Armstrong BA, Perez CA, Simpson JR, Hederman MA. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 1987;13:531вҖ“9.
9. Chan RH, Dar AR, Yu E, et al. Superior vena cava obstruction in small-cell lung cancer. Int J Radiat Oncol Biol Phys 1997;38:513вҖ“20.
10. Lonardi F, Gioga G, Agus G, Coeli M, Campostrini F. Double-flash, large-fraction radiation therapy as palliative treatment of malignant superior vena cava syndrome in the elderly. Support Care Cancer 2002;10:156вҖ“60.
11. National Comprehensive Cancer Network. Non-small cell lung cancer (Version 3.2023) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; c2023 [cited 2023 Sep 6]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
12. Yu JB, Wilson LD, Detterbeck FC. Superior vena cava syndrome: a proposed classification system and algorithm for management. J Thorac Oncol 2008;3:811вҖ“4.
13. Nichols L, Saunders R, Knollmann FD. Causes of death of patients with lung cancer. Arch Pathol Lab Med 2012;136:1552вҖ“7.
14. Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi R. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol 2009;71:343вҖ“9.
15. Leon D, Rao S, Huang S, et al. Literature review of percutaneous stenting for palliative treatment of malignant superior vena cava syndrome (SVCS). Acad Radiol 2022;29 Suppl 4:S110вҖ“20.
16. Nguyen NP, Borok TL, Welsh J, Vinh-Hung V. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax 2009;64:174вҖ“8.
17. Friedman T, Quencer KB, Kishore SA, Winokur RS, Madoff DC. Malignant venous obstruction: superior vena cava syndrome and beyond. Semin Intervent Radiol 2017;34:398вҖ“408.
18. Kapur S, Paik E, Rezaei A, Vu DN. Where there is blood, there is a way: unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics 2010;30:67вҖ“78.
19. Stephens RJ, Hopwood P, Girling DJ. Defining and analysing symptom palliation in cancer clinical trials: a deceptively difficult exercise. Br J Cancer 1999;79:538вҖ“44.
20. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798вҖ“804.
21. Olafsdottir HS, Klevebro F, Ndegwa N, Alexandersson von Dobeln G. Short-course compared to long-course palliative radiotherapy for oesophageal cancer: a single centre observational cohort study. Radiat Oncol 2021;16:153.
22. Rades D, Segedin B, Conde-Moreno AJ, et al. Radiotherapy with 4 Gy Г— 5 versus 3 Gy Г— 10 for metastatic epidural spinal cord compression: final results of the SCORE-2 Trial (ARO 2009/01). J Clin Oncol 2016;34:597вҖ“602.
23. Kramer GW, Wanders SL, Noordijk EM, et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 2005;23:2962вҖ“70.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |